0496
Accelerated dynamic quantitative perfusion imaging using an optimized simultaneous multi-slice (SMS) spin and gradient echo (SAGE) sequence with joint-virtual coil (JVC) reconstruction1MGH Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, United States, 2Department of Biomedical Engineering, Zhejiang University, Hangzhou, China
Synopsis
A 5-echo spin and gradient echo (SAGE) sequence was implemented with SMS to provide whole brain coverage at a high spatio-temporal resolution during DSC imaging. Complementary k-space sampling between echoes and joint-virtual coil (JVC) reconstruction were used to achieve high quality reconstruction at 9x acceleration, which enabled 1.9x1.9x5 mm whole-brain imaging at a TR of 1.6s. The multi-echo images from this sequence were fit to achieve quantitative R2 and R2* maps for each TR. This has been shown to limit T1 leakage effects and provide more accurate and detailed perfusion measures with the potential to better assess tumor diagnostics and progression.
Introduction
Dynamic susceptibility contrast (DSC) perfusion imaging gives quantitative measures such as cerebral blood flow/volume (CBF/CBV) by examining the transient perfusion dynamics of a bolus of contrast agent injection. Recent studies have shown that measurements of both spin and gradient echo images may help to improve diagnoses1–3, as they are sensitive to micro-vasculature and macro-vasculature changes, respectively. The spin-and-gradient echo (SAGE)4 sequence acquires multiple echoes with a mixture of spin and gradient echo contrasts that can be used to simultaneously measure R2 and R2* by fitting the images to the signal equation5. Measuring R2/R2* directly reduces the need for T1-leakage correction and/or a preload, and allows for a more accurate calculation of the arterial input function (AIF)6, particularly in tumor patients with a compromised blood brain barrier.
While SAGE greatly improves signal quantitation and provides exciting new vasculature information7, its added encoding burden hinders the ability for DSC to achieve sufficient resolution and volume coverage at an adequate sampling rate to capture the passage of contrast agent through a tumor. Therefore, SAGE acquisitions are usually limited in slice number and spatial and/or temporal resolution, making it difficult to visualize tumor activity and vascular changes. In this work, we implement a modified-SAGE sequence with a blipped-CAIPI-SMS acquisition8 to allow for whole-brain coverage with a high temporal and spatial resolution. Complementary k-space sampling between echoes and joint-virtual coil (JVC)-GRAPPA reconstruction9–11 were employed to achieve accelerated high quality reconstruction.
Methods
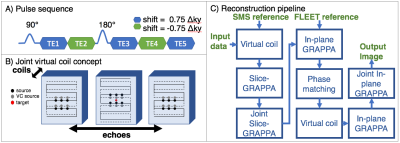
A DSC scan with a 5-echo SAGE sequence (Fig.1A) was acquired (Siemens Skyra 3T) on a healthy subject during a gadolinium injection. Sequence parameters include: in-plane resolution=1.9x1.9mm, FOV=220x220mm, slice thickness=5mm, 27 slices, TR=1.6s, TEs=[18, 47, 75, 104, 132]ms, with a total acceleration factor=9 (MB=3,Rinplane=3). K-space sampling was shifted across echoes by 0.75$$$\Delta$$$ky and -0.75$$$\Delta$$$ky to increase coverage for joint-GRAPPA and offset from the center to allow for more effective virtual coil sampling, as shown in Fig.1B. Total scan time was 3.2min (1min FLEET12 training data, 2.2min acquisition).
Both standard and joint-virtual coil (JVC) reconstructions (Fig.1C) were performed in Matlab to assess their comparative performance (online reconstruction via standard methods are also available in our implementation of this sequence). JVC-GRAPPA uses the phase prior in virtual coil concept9 as well as joint information across multiple contrasts to reconstruct each image. The joint reconstruction was initialized by using the reconstructed k-space from standard GRAPPA as training data, with a window of three echoes.
The 5-echo images were motion corrected across the time series and masked using FSL13. The images were then used to calculate R2/R2* maps by performing a four-parameter fit to the signal equation4. Subsequently, the R2/R2* maps were processed with NordicICE (NordicNeuroLab, Norway) to generate CBF and CBV maps.
Results
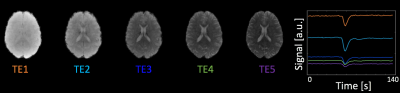
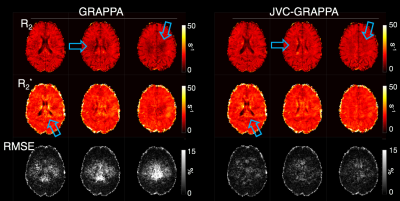
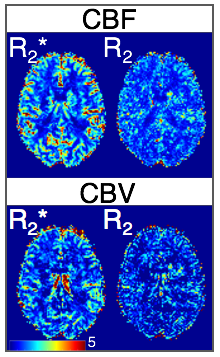
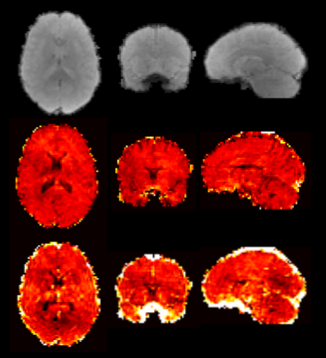
Fig.2 shows 5-echo images from representative slice, as well as the mean slice signal over the time series in all echoes. Fig.3 shows R2/R2* maps for both reconstructions from a baseline TR, with the RMSE of the fit shown below. The JVC maps show substantial improvement compared to the standard reconstructed images, allowing an acceleration factor of 9-fold with relaxation rates comparable to published values5,14,15. Fig.4 shows normalized CBF/CBV maps obtained from the time-series of the R2/R2* maps in the JVC reconstruction.Discussion
The presented method gives quantitative R2 and R2* maps with whole-brain coverage at a TR of 1.6s, which can be used to calculate quantitative perfusion parameters directly to eliminate the need for T1 leakage correction and/or preload injection. Shown here as a proof of concept are relative CBV and CBF maps in a healthy subject, however one advantage of the SAGE sequence is the ability to calculate the AIF directly from the R2* map and achieve absolute measures of CBV/CBF, which would further aid in tumor assessment. In addition, the data from this sequence can be used to decouple the T2* and T1 signal components to measure DCE permeability, with the inclusion of a pre-bolus T1 map7,16. These potential benefits will be explored in our planned work on a tumor population.
In this work, JVC was used to achieve good reconstruction at a high 9x acceleration. The benefit of this acceleration is not limited to the chosen protocol but can be flexibly used to improve other DSC protocols such as an example 2.9mm isotropic acquisition case in Fig.5. In future studies, other advanced reconstruction methods such as LORAKS17 and joint-LORAKS will be explored for improved exploitation of joint/prior information across echoes and TRs to allow further accelerations. To this end, temporal information and complementary sampling across TRs could represent an interesting avenue of investigation.
Acknowledgements
This work was supported in part by NIH research grants: R01EB020613, R01EB019437, R24MH106096, P41EB015896, and the shared instrumentation grants: S10RR023401, S10RR019307, S10RR019254, S10RR023043.References
1. Donahue KM, Krouwer HGJ, Rand SD, et al. Utility of simultaneously acquired gradient-echo and spin-echo cerebral blood volume and morphology maps in brain tumor patients. Magn Reson Med. 2000;43(6):845-853. doi:10.1002/1522-2594(200006)43:6<845::AID-MRM10>3.0.CO;2-J.
2. Speck O, Chang L, DeSilva NM, Ernst T. Perfusion MRI of the human brain with dynamic susceptibility contrast: Gradient-echo versus spin-echo techniques. J Magn Reson Imaging. 2000;12(3):381-387. doi:10.1002/1522-2586(200009)12:3<381::AID-JMRI2>3.0.CO;2-Y.
3. Kiselev VG, Strecker R, Ziyeh S, Speck O, Hennig J. Vessel size imaging in humans. Magn Reson Med. 2005;53(3):553-563. doi:10.1002/mrm.20383.
4. Schmiedeskamp H, Straka M, Newbould RD, et al. Combined spin- and gradient-echo perfusion-weighted imaging. Magn Reson Med. 2012;68(1):30-40. doi:10.1002/mrm.23195.
5. Schmiedeskamp H, Straka M, Bammer R. Compensation of Slice Profile Mismatch in Combined Spin- and Gradient-Echo Echo-Planar Imaging Pulse Sequences. Magn Reson Med. 2012;67:378-388. doi:10.1002/mrm.23012.
6. Newton AT, Pruthi S, Stokes AM, Skinner JT, Quarles CC. Improving perfusion measurement in DSC-MR imaging with multiecho information for arterial input function determination. Am J Neuroradiol. 2016;37(7):1237-1243. doi:10.3174/ajnr.A4700.
7. Schmiedeskamp H, Andre JB, Straka M, et al. Simultaneous Perfusion and Permeability Measurements Using Combined Spin- and Gradient-Echo MRI. J Cereb Blood Flow Metab. 2013;33(5):732-743. doi:10.1038/jcbfm.2013.10.
8. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210-1224. doi:10.1002/mrm.23097.
9. Blaimer M, Gutberlet M, Kellman P, Breuer FA, Köstler H, Griswold MA. Virtual coil concept for improved parallel MRI employing conjugate symmetric signals. Magn Reson Med. 2009;61(1):93-102. doi:10.1002/mrm.21652.
10. Chen F, Shi X, Chen S, et al. Accelerated model-based proton resonance frequency shift temperature mapping using echo-based GRAPPA reconstruction. Magn Reson Imaging. 2015;33(2):240-245. doi:10.1016/j.mri.2014.10.006.
11. Ramb R, Mader I, Jung B, Hennig J, Zaitsev M. High resolution CBV assessment with PEAK-EPI: k-t-undersampling and reconstruction in echo planar imaging. Magn Reson Med. 2017;77(6):2153-2166. doi:10.1002/mrm.26298.
12. Polimeni JR, Bhat H, Witzel T, et al. Reducing sensitivity losses due to respiration and motion in accelerated echo planar imaging by reordering the autocalibration data acquisition. Magn Reson Med. 2016;75(2):665-679. doi:10.1002/mrm.25628.
13. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(SUPPL. 1):S208-S219. doi:10.1016/j.neuroimage.2004.07.051.
14. Stanisz GJ, Odrobina EE, Pun J, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005;54(3):507-512. doi:10.1002/mrm.20605.
15. Li T-Q, Yao B, van Gelderen P, et al. Characterization of T 2 * heterogeneity in human brain white matter. Magn Reson Med. 2009;62(6):1652-1657. doi:10.1002/mrm.22156.
16. Stokes AM, Skinner JT, Yankeelov T, Quarles CC. Assessment of a simplified spin and gradient echo (sSAGE) approach for human brain tumor perfusion imaging. Magn Reson Imaging. 2016;34(9):1248-1255. doi:10.1016/j.mri.2016.07.004.
17. Haldar JP. Low-Rank Modeling of Local k-Space Neighborhoods (LORAKS) for Constrained MRI. IEEE Trans Med Imaging. 2014;33(3):668-681. doi:10.1109/TMI.2013.2293974.
Figures