0495
The effects of diffusion on the tissue orientation dependent gradient echo dynamic susceptibility contrast signal1UBC MRI Research Centre, University of British Columbia, Vancouver, BC, Canada, 2Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada, 3Pediatrics, University of British Columbia, Vancouver, BC, Canada, 4Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 5Division of Neurology, Faculty of Medicine, University of British Columbia, Vancouver, BC, Canada
Synopsis
Gradient echo dynamic susceptibility contrast (DSC) imaging of cerebral white matter tissue perfusion exhibits a strong dependency on the angle between white matter fibres and the main magnetic field. Here, we investigate the extent to which the diffusion of spins within the locally magnetically inhomogenous environment affects the orientation dependency of the DSC signal using a numerical model of the transverse magnetization within a voxel containing both isotropic and anisotropic vasculature. We found that diffusion increases the measured change in $$$R_2^*$$$ by up to an average of 3.2% for typical diffusion values, independent of fibre orientation, and further improves the fit between the simulated and measured $$$R_2^*$$$ values.
Introduction
Perfusion in cerebral white matter tissue as measured by gradient echo dynamic susceptibility contrast (DSC) imaging is known to exhibit a strong dependency on the angle between the white matter (WM) fibre and the main magnetic field $$$B_0$$$1. This effect is due to large anisoptropic vasculature running in parallel with the WM tracts, which introduces orientation dependent magnetic field inhomogeneities that are superimposed on top of orientation independent field inhomogeneities due to isotropic vasculature. This results in the measured change in $$$T_2^*$$$ during a DSC experiment to depend on the angle between the WM tract and $$$B_0$$$, as well as the contrast agent (CA) concentration. In particular, field inhomogeneities near large anisotropic vessels increase with increasing fibre angle and increasing CA. This causes a large orientation effect in measured $$$\Delta{R_2^*}$$$ values, on the order of 100%1.
While a model based on static dephasing fits the measured data well, diffusion of spins within the static magnetic field inhomogeneities introduces further signal loss. This is due to spins being exposed to larger field inhomogeneities in the vicinity of blood vessels by means of diffusion, effectively extending the range of the inhomogeneities; this effect is further increased by the CA. The orientation dependency of the signal loss due to diffusion depends on the relative amount of isotropic vs. anisotropic vasculature; diffusive signal loss near smaller vessels is larger due to higher field gradients, and thus the isotropic losses can dominate. Here, we investigate the extent to which diffusion impacts the measured $$$\Delta{R_2^*}$$$ value as a function of WM fibre orientation in GRE-DSC through an extension of a previously developed numerical model of diffusion within a magnetically inhomogeneous environment consisting of a vascular bed with both isotropic and anisotropic components3.
Methods
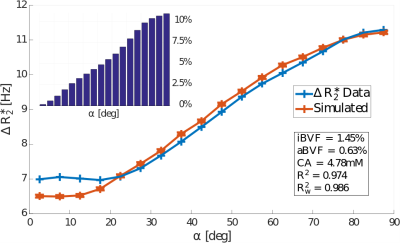
Data from 13 subjects with multiple sclerosis was acquired at 3T1. DSC was acquired with gradient echo EPI (TR/TE=2417ms/40ms, voxel volume=1.5x1.5x4$$${mm}^3$$$). Fibre angle was calculated using a DTI scan as described in2. The DSC signal in each WM voxel was sorted according to fibre angle $$$\alpha$$$ into bins of width $$$5^\circ$$$ ranging from $$$2.5^\circ$$$ to $$$87.5^\circ$$$. $$$\Delta{R_2^*}=-\frac{1}{TE}\log(\frac{S}{S_0})$$$ was calculated and averaged across all voxels in each bin for all subjects. $$${S_0}$$$ is the baseline signal, and $$${S}$$$ is the signal corresponding to peak CA. Figure 1 shows the resulting $$$\Delta{R_2^*}$$$ vs. $$$\alpha$$$ curve, as well as the distribution of voxels with respect to fibre angle.
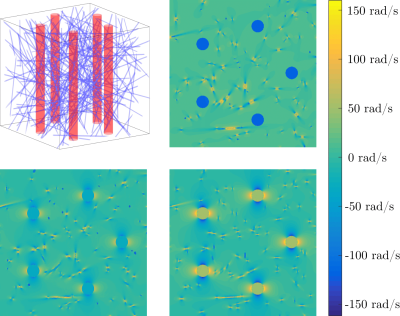
Simulation of the transverse magnetization occurs within a voxel filled with anisotropic and isotropic blood vessels. The construction of the voxel geometry and subsequent calculation of field inhomogeneities is described in3. An example voxel and corresponding field inhomogeneity cross-sections are shown in Figure 2. Within this geometry, the transverse magnetization is propagated forward in time by solving the Bloch-Torrey equation $$$\frac{dM}{dt}=D{\nabla^2}M-(R_2(\vec{r})+i\omega(\vec{r}))M$$$ up until the echotime TE=40ms3.The simulation contains three free parameters which are varied in order to best reproduce the measured $$$\Delta{R_2^*}$$$ vs. $$$\alpha$$$ curve: the isotropic blood volume fraction iBVF of the small isotropic vessels, the anisotropic blood volume fraction aBVF of the large anisotropic vessels, and the peak contrast agent concentration CA. These three parameters were optimised using weighted least squares regression with MATLAB's lsqnonlin function, where the weights were taken to be the number of data points in each angle bin in order to account for the angular distribution of the WM fibres (Figure 1). Additionally, the diffusion constant and number of large vessels simulated were varied in discrete steps.Results
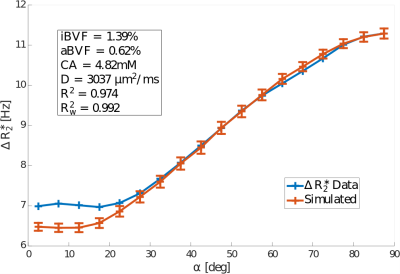
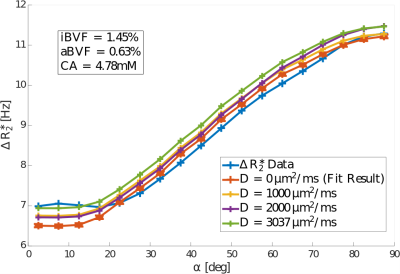
Diffusion was found to increase the modelled $$$\Delta{R_2^*}$$$ value by up to 0.22Hz, 0.25Hz, and 0.39Hz for diffusion constants of $$$1mm^2/s$$$, $$$2mm^2/s$$$, and $$$3.037mm^2/s$$$3, respectively, independent of fibre orientation (Figure 4). The corresponding weighted average increases in $$$\Delta{R_2^*}$$$ were 1.4%, 1.9%, and 3.2%. Optimal model fits were obtained for N=4 anisotropic vessels. The inclusion of diffusion improved the model fit, with the weighted coefficient of determination $$$R^2_w$$$ increasing from 0.986 to 0.992 (Figure 3).Discussion and Conclusion
Diffusion was found to increase the simulated $$$\Delta{R_2^*}$$$ value approximately independent of the orientation of the WM fibre with respect to $$$B_0$$$, suggesting that diffusive signal loss is dominated by isotropic vasculature as opposed to anisotropic vasculature for GRE-DSC scans. This finding agrees well with the optimised parameter values of iBVF=1.39% and aBVF=0.62%, indicating that approximately two-thirds of WM vasculature is contained in the isotropic vessels. A total blood volume fraction of 2.01% was found, which agrees well with literature values.
Additionally, including diffusion increased the weighted coefficient of determination $$$R^2_w$$$ of the model. This suggests that diffusion plays a small, but not insignificant role in GRE-DSC imaging compared to static dephasing alone. However, the parameters computed with the extended model are very similar to the static dephasing model.
Acknowledgements
No acknowledgement found.References
1. Hernández-Torres E, Kassner N, Forkert ND, Wei L, Wiggermann V, Daemen M, Machan L, Traboulsee A, Li D, Rauscher A. Anisotropic cerebral vascular architecture causes orientation dependency in cerebral blood flow and volume measured with dynamic susceptibility contrast magnetic resonance imaging. J Cereb Blood Flow Metab. 2016
2. Hernández-Torres E, Wiggermann V, Hametner S, et al. Orientation dependent MR signal decay differentiates between people with MS, their asymptomatic siblings and unrelated healthy controls. PLoS One 2015; 10: e014095
3. Jonathan Doucette, Luxi Wei, Christian Kames, et al. Anisotropic cerebral vascular architecture causes orientation dependency in cerebral blood flow and volume measured with spin echo dynamic susceptibility contrast magnetic resonance imaging. Proceedings of the International Society of Magnetic Resonance and Medicine 25, Honolulu, Hawaii, USA.
Figures