0484
Antibiotic treatment affects vascular elastin remodeling both focally and distally to the site of aortic injury in a murine model1School of Biomedical Engineering Imaging Sciences, King's College London, London, United Kingdom, 2Radiology department, School of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile
Synopsis
Atherosclerosis is a systemic, inflammatory disease of the large and medium-sized arteries. Although vascular interventions aim at treating focal stenosis, they may trigger systemic responses that accelerate lesions elsewhere. Elastin remodeling plays a crucial role in vessel wall thickening with monocytes and vascular smooth muscle cells (VSMC) being the primary sources of elastin synthesis. In this study, we used an elastin-binding MR contrast agent to assess whether (1) vascular injury in the abdominal aorta accelerates atherosclerosis in the brachiocephalic artery located distally to the site of injury (2), whether antibiotic treatment alters vascular elastin remodeling and (3) whether antibiotic treatment alters VSMC migration and proliferation and monocyte recruitment and polarization.
Introduction
Intimal thickening occurs in blood vessels in response to focal mechanical injury or atherosclerosis. Intimal thickening is associated with activation of vascular smooth muscle cells (VSMCs) and increased recruitment of monocytes, that affect extracellular matrix remodeling1. Minocycline is a tetracycline derivate with cardioprotective effects independent of its antimicrobial properties2-4. We have previously shown that late gadolinium enhancement (LGE) MRI using an elastin-specific binding contrast agent (ESMA) provides information on elastin remodeling in animal models of cardiovascular diseases5-7. Here, we investigated the merits of Gd-ESMA to assess in vivo (1) whether focal vascular injury accelerates atherosclerosis in segments located distally to the site of injury, (2) whether minocycline affects elastin remodeling both focally, at the site of aortic injury, and distally in the brachiocephalic artery (BCA) and (3) whether minocycline treatment has a direct effect on VSMCs (migration and proliferation) and monocyte recruitment and polarization.Methods
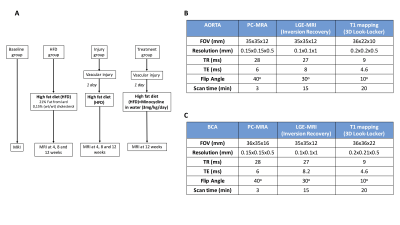
Study design is described in Fig 1A. Animal surgical model: Aortic endothelial injury was performed as described previously8. In-vivo MRI: A 3T MR scanner (Philips Achieva, Best, NL) equipped with a 47mm (aorta) and a 23mm (brachiocephalic artery - BCA) single-loop microscopy surface coil was used. Images were acquired 2 hours after intravenous administration of an elastin-binding contrast agent (ESMA) (0.2mmol/kg). Acquisition parameters for the aorta and BCA are summarized in Fig 1B-1C, respectively. Flow cytometry: Monocyte characterization was performed using specific antibodies for F4/80, CD115 and Ly6C. Histology: Verhoeff Van Gieson elastic stain and tropoelastin immunohistochemistry (IHC) were used to examine elastin remodeling and Ki67 IHC was used for assessment of cell proliferation. In vitro: Migration of primary VSMCs was evaluated by using the scrape-injury model and proliferation using Ki67 immunofluorescence.Results
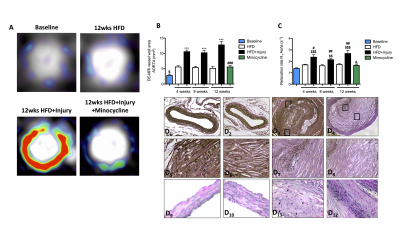
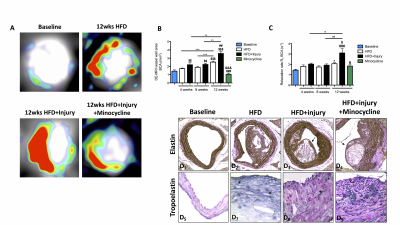
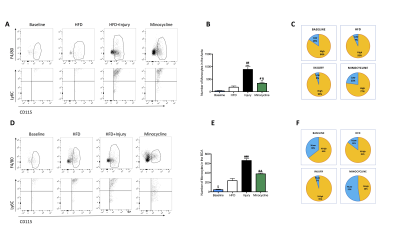
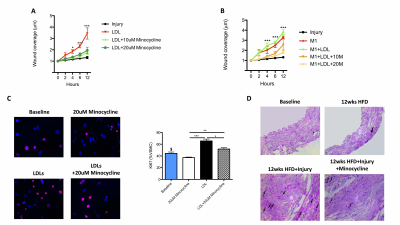
LGE-MR images of the aorta after injection of Gd-ESMA are shown in Fig 2A. Increased contrast uptake (Fig 2B) and aortic (R1) vessel wall relaxation rate (Fig 2C) were observed in mice fed a high-fat-diet (HFD) that had undergone vascular injury compared to the HFD and control groups. Importantly, HFD-injured mice treated with minocycline showed decreased Gd-ESMA uptake, suggesting a reduction of elastin remodeling (Fig 2B-2C) compared with untreated mice. Histology (Fig 2D) showed elastin remodeling in the HFD-injury group (Fig 2E3) but not in minocycline-treated group (Fig 2E4). To investigate the systemic effect of focal vascular injury on distal atherosclerosis progression we evaluated plaque formation in the brachiocephalic artery (BCA). LGE-MRI images of the BCA after injection of Gd-ESMA are shown in Fig 3A. Greater enhancement and increased R1 relaxation rate was detected in the BCA (Fig 3B) in the HFD-injury group compared with HFD alone and the control groups (Fig 3C), suggesting that vascular injury accelerates atherosclerosis in distal vessel segments compared with HFD alone. Mice treated with minocycline showed decreased Gd-ESMA uptake (Fig 3B-3C) compared to the untreated groups. Histological analysis showed increased elastin remodeling in the HFD-injury group compared with the HFD group (Fig 3E2 and 3E3) but reduced elastin remodeling in the minocycline-treated group (Fig 3E4). To understand the cellular effects of minocycline, we analysed tissue monocytes by flow cytometry (Fig 4A and 4D). HFD-injured mice showed increased recruitment of inflammatory Ly6Chigh monocytes in both the aorta and distally in the BCA compared with HFD mice. When injured mice were treated with minocycline they showed significantly less monocyte recruitment in both vascular segments compared to the untreated mice (Fig 4B and 4E). Importantly, there was a shift in monocyte subpopulations, from Ly6Chigh to Ly6Clow in the aorta and an even more pronounced shift in the BCA after minocycline-treatment (Fig 4C and 4F). Additionally, we evaluated the effect of minocycline on the migration and proliferation of VSMCs. In vitro VSMC migration was stimulated when the cells were treated with low density lipoproteins (LDL), an inflammatory-rich medium collected from M1 macrophages or the combination of both. However, VSMC migration was reduced when cells were treated with minocycline (Fig 5A-5B), suggesting that minocycline decreases the inflammatory response triggered by LDL and/or M1 macrophages. Finally, minocycline reduced the percentage of VSCM proliferation both in vitro (Fig 5C) and in vivo (Fig 5D).Conclusion
We demonstrate that focal vascular injury accelerates atherosclerosis in distal vessel segments through a systemic response driven by monocytes. Minocycline treatment alters elastin remodeling and retards intimal thickening both at the site of vascular injury and distally by changing the phenotype of both monocytes and VSMCs.Acknowledgements
No acknowledgement found.References
1Dimitry, A. C. Cardiology in review. 2013; 2Ohshima, S. JACC. 2010. 3Shahzad, K. Atherosclerosis. 2011. 4Phinikaridou A. J Am Heart Assoc. 2013. 5Makowski, M.R. Nature Medicine. 2011. 6Botnar, R.M. Circ Cardiovasc Imaging. 2014. 7Protti, A. J Am Heart Assoc. 2015. 8Lavin, B. Circ Cardiovasc Imaging. 2015.Figures