0483
Relationship between MR characteristics of peripheral artery lesions and difficulty of peripheral endovascular procedures1Schulich Heart Program and the Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 2Division of Vascular Surgery, Department of Surgery, University of Toronto, Toronto, ON, Canada, 3Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
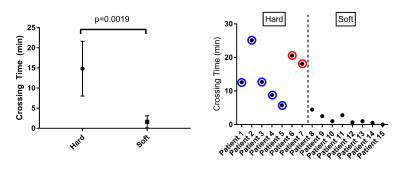
The most common mode of failure for percutaneous vascular interventions (PVI) is the inability to cross hard lesions with a guidewire. This study uses magnetic resonance (MR) lesion characterization to predict the difficulty of PVI. Steady state free precession (SSFP) MR angiography and ultrashort echo time imaging were used to categorize lesions as “hard” (e.g. calcium, collagen), or “soft” (thrombus, lipids). 17 patients were imaged prior to PVI. MRI-defined hard lesions required significantly longer time to cross (14.81 min vs 1.61 min) and required stenting more often.
Introduction
Percutaneous vascular interventions (PVI) are associated with high technical failure and re-intervention rates.1 Limitations with current peripheral artery imaging modalities make patient selection for PVI difficult. The most common mode of PVI failure is the inability to cross a lesion that is too hard or too stiff. In this study, we use MRI to characterize peripheral artery lesions to predict whether lesions are difficult to cross with a guidewire, and therefore at high risk of failure.Methods
17 patients with peripheral artery disease underwent MR imaging with two research sequences prior to their PVI. A novel steady-state free precession (SSFP) flow-independent MR angiogram was used to locate lesions, and an ultrashort echo time (UTE) subtraction sequence was used to identify hard lesions composed of calcium and collagen.2 The 3D SSFP MR angiogram used the following parameters: Water-selective excitation; Field of view (FOV) 24 x 24 x 24 cm3; image resolution 1 x 1 x 1 mm3; repetition time (TR) 5.54 ms; echo time (TE) 3.58 ms; flip angle 45°; number of averages 1; acquisition time 2 mins. The UTE imaging was performed using a prototype 3D Cones sequence from GE Healthcare with the following parameters: FOV 18 x 18 x 10 cm3; image resolution 1 x 1 x 1 mm3; TR 10 ms, TE1 30 μs, TE2 2.25 ms; flip angle 9°; number of averages 1; acquisition time 5 mins. 2 patient image sets were used to train 2 independent blinded physician reviewers, and they categorized the remaining 15 lesions based on their MR imaging signatures. Lesions were characterized as “hard” if ≥50% of the lumen was opacified in the UTE subtraction image (signal at TE1 – signal at TE2) in the hardest cross-section within the lesion. A third independent blinded reviewer scored the lesions of the superficial femoral artery (SFA) based on x-ray angiography (the current gold standard) with the TASC II classification scheme.3 The primary and secondary outcomes were the time required to cross the target lesion with a guidewire and the need for stenting, respectively.Results
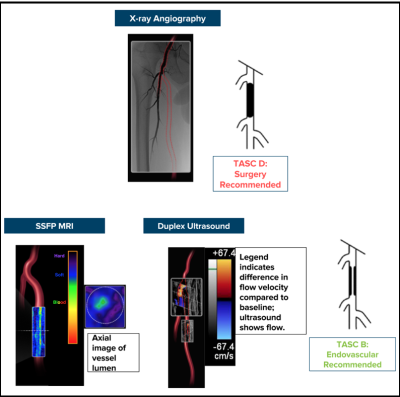
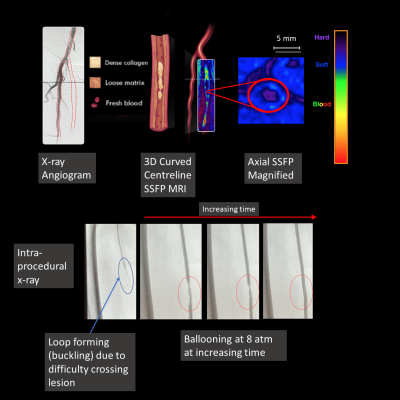
2/17 data sets were used for image analysis training purposes. 11/15 lesions were in the SFA. 7/15 (47%) of the lesions were categorized as “hard” and 8/15 (53%) of the lesions were “soft” based on MR image characteristics. 2/15 could not be crossed with a guidewire and both lesions were hard. The 5/7 hard lesions that could be crossed required significantly more time compared with soft lesions (14 min 49 sec ± 6 min 48 sec vs 1 min 37 sec ± 1 min 30 sec (two-tailed t-test, p=0.0019)) (Fig 1). All 5 of the hard lesions that could be crossed required stenting; no soft lesion required stenting (Fisher’s exact test, p=0.0008). All 5 hard lesions could only be crossed in the subintimal plane, but only 2/8 soft lesions required subintimal crossing (Fisher’s exact test, p=0.021). There were no significant differences in crossing time using TASC classifications (TASC A/B: 3 min 58 sec ± 5 min 21 sec vs TASC C/D lesions: 13 min 20 sec ± 9 min 34 sec, p = 0.0992). Of note, 3/7 hard lesions and 5/8 soft lesions were angiographic total occlusions but had patent channels on the flow-independent SSFP images (Fig 2). Also, 4/7 hard lesions had little to no calcium and were not seen on x-ray angiography (Fig 3).Discussion
The percutaneous treatment of peripheral artery disease is growing exponentially.4 Informed patient selection is necessary due to the high failure rates of PVI. The TASC guidelines are the most widely used classification system5 to determine which patients are suitable for PVI based on x-ray angiographic anatomic characteristics. We found MR lesion classification could identify patients with lesions that took longer to cross while TASC classification did not. One issue with classifying lesions using x-ray angiography is that up to 20% of vessels seen on MRI are occult on x-ray6 (Fig 2). In addition, minimally calcified hard lesions (composed of dense collagen) also required long crossing times but cannot be seen on x-ray angiography (Fig 3).Conclusion
MRI can be used to determine which peripheral artery lesions are more difficult to cross with a guidewire. This may facilitate more informed patient selection compared with clinically available classification schemes. Future work will determine if MRI lesion characterization can predict long-term endovascular outcomes to aid procedural planning.Acknowledgements
This study was funded by the Canadian Institutes of Health Research (MOP-126169).References
1. Bradbury AW, Adam DJ, Beard JD, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): Multicentre, randomised controlled trial. Lancet. 2005;366(9501):1925-1934. doi:10.1016/S0140-6736(05)67704-5.
2. Roy T, Liu G, Shaikh N, Dueck AD, Wright GA. Puncturing Plaques : Relating MRI Characteristics of Peripheral Artery Lesions to Guidewire Puncture Forces. J Endovasc Ther. 2017;24(1):35-46. doi:10.1177/1526602816671135.
3. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the management of peripheral arterial disease (TASC II). Int Angiol. 2007;26(2):82-157. doi:10.1016/j.jvs.2006.12.037.
4. Anderson PL, Gelijns A, Moskowitz A, et al. Understanding trends in inpatient surgical volume: Vascular interventions, 1980-2000. J Vasc Surg. 2004;39(6):1200-1208. doi:10.1016/j.jvs.2004.02.039.
5. Stoner MC, Calligaro KD, Chaer RA, et al. Reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J Vasc Surg. 2016;64(1):e1-e21. doi:10.1016/j.jvs.2016.03.420.
6. Owen RS, Carpenter JP, Baum RA, Perloff LJ, Cope C. Magnetic resonance imaging of angiographically occult runoff vessels in peripehral arterial occlusive disease. N Engl J Med. 1992;326(24):1577-1581.
Figures