0482
CAPTURE: Consistently-Acquired Projections for Tuned and Robust Estimation - A Self-Navigated Respiratory Motion Correction Approach1Washington University in St. Louis, St. Louis, MO, United States
Synopsis
In this study, we present a fully-automated and robust self-navigated approach to obtain 4D motion-resolved images during free breathing. A 1D navigator was acquired consistently in order to reduce the signal contamination due to system imperfections. The resulting projections were then 'tuned' using complex phase rotation for adapting to scan-to-scan variations, followed by the detection of the respiratory curve. CAPTURE successfully detected respiratory motion in all subjects. A blind radiological review by two radiologists shows the significant quality improvement in motion-corrected images.
Introduction
Respiratory motion constitutes a challenging problem in body MRI. Radial acquisitions offer self-navigation via the fluctuations observed in the k-space data with the expectation that respiration is going to induce changes in these data1-4. However, k-space trajectories can be shifted away from their presumed locations due to gradient delays and eddy currents5, introducing large non-physiological variations that can easily contaminate and shadow respiratory content. Moreover, due to inter-subject and inter-scan variability, devising a robust method that can successfully detect respiratory motion in all cases is difficult6.
To address these issues, we present a new approach termed as Consistently-Acquired Projections for Tuned and Robust Estimation (CAPTURE). Our method is robust to gradient imperfections and can adapt to inter-subject and inter-scan variations.
Methods
CAPTURE is a variant of the stack-of-stars sequence and has two features:
1) Consistently-Acquired Projections [CAP-]:
A 1D navigator is consistently acquired at a fixed azimuthal angle for all stacks of spokes in order to reduce non-physiological signal contamination.
2) Tuned and Robust Estimation [-TURE]:
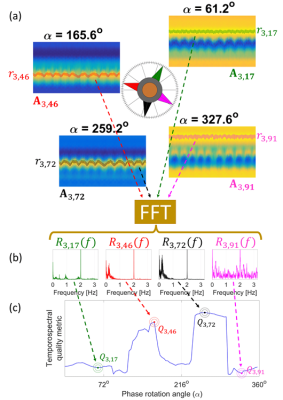
The navigator k-space data are first apodized using a Hamming window. After a 1D inverse Fourier Transform, the resulting complex projections from all stacks of spokes received through Coil $$$i$$$ are placed as columns of a 2D matrix, $$$\textbf{P}_i = \textbf{P}_i[x,n]$$$, where $$$x$$$ is the index for the physical locations on the superior-inferior axis and $$$n$$$ is the stack-of-spokes index. A ‘tuning’ process is then performed by phase-rotating $$$\textbf{P}_i$$$’s over the full phase range, (0°,360°], via 100 different complex scalar multiplications $$$e^{-j\alpha_m}\textbf{P}_i$$$, where $$$\alpha_m=m \times 3.6^o$$$ with $$$m=1,2,...,100$$$. For each $$$\alpha_m$$$, a respiratory curve $$$r_{i,m}[n]$$$ is obtained by detecting the location of the peak along each column of $$$\textbf{A}_{i,m} \triangleq Re\{e^{-j\alpha_m}\textbf{P}_i\}$$$, where $$$Re\{.\}$$$ is the real part operator. The detected respiratory curves, $$$r_{i,m}[n]$$$, are Fourier-transformed, yielding $$$R_{i,m}(f)$$$. A tempo-spectral quality metric is then used for selecting the best curve:
$$Q_{i,m} = \frac{\int^{0.5 Hz}_{0.1 Hz} \left|R_{i,m}(f)\right| df}{\int^{\infty}_{0.8 Hz} \left|R_{i,m}(f)\right| df}\times \left(\frac{1}{\max_{n}r_{i,m}[n]-\min_{n}r_{i,m}[n]}\right)$$
The first term in the product favors respiratory content over system-related spectral contamination, whereas the second term penalizes the peak-to-trough range of the detected curve as a precaution for signal jumps. The best coil and the best phase-rotation for that coil are chosen according to: $$\left(i^*,m^*\right)=\arg\max_{\left(i,m\right)}Q_{i,m}$$
Figure 1 illustrates the detection scheme.
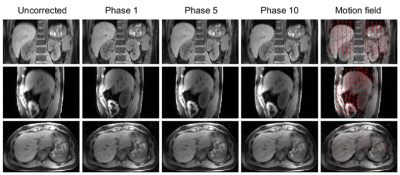
A low-pass filter with a cut-off frequency of 1 Hz is used for removing residual and physiologically irrelevant contamination. The k-space data are then binned into 10 respiratory phases using the detected curve and are later reconstructed using a compressed sensing method similar to XD-GRASP4, with an additional spatial smoothness constraint.
HIPAA-compliant experiments with an approved IRB protocol were run on 10 healthy participants (TE/TR = 1.69ms/3.54ms, Voxel size = 1.125x1.125x3 mm3) and 12 liver tumor patients. The CAPTURE-corrected and uncorrected images were scored blindly by two independent radiologists.
Results
Respiratory motion frequencies of the 22 participants ranged from 0.14 to 0.52 Hz whereas the craniocaudal motion ranged from 10.1 to 34.9 mm in the diaphragm. Moreover, the respiratory patterns varied greatly across participants and also within the same imaging session for some participants. Despite this high variability, CAPTURE was able to extract respiratory motion successfully for all datasets in a fully-automated manner.
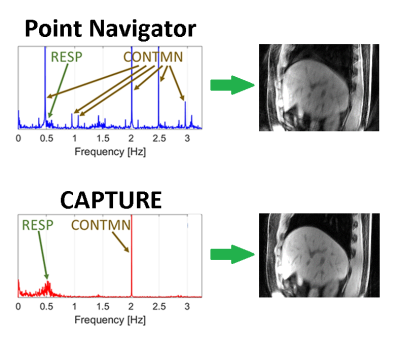
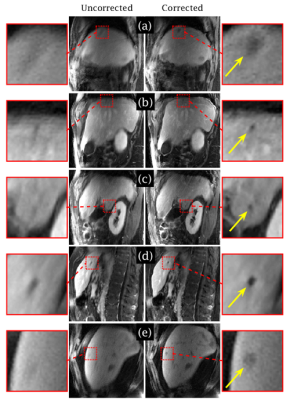
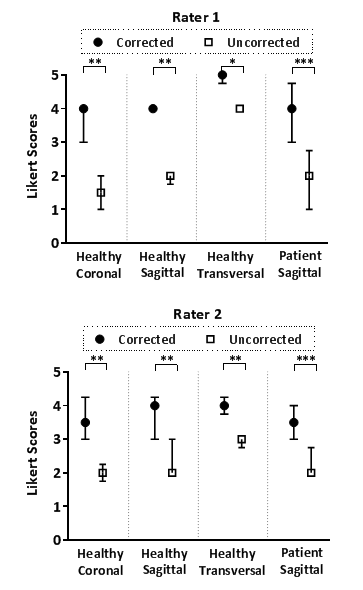
Figure 2 compares the spectra obtained by the k-space center point navigator versus CAPTURE for a healthy subject. The curve detected by CAPTURE has stronger respiratory content and a much cleaner frequency spectrum with only one non-physiological peak at about 2 Hz. As Figure 3 shows, CAPTURE can resolve respiratory motion and improve image sharpness regardless of the imaging orientation. Figure 4 exhibits that lesions became more conspicuous in CAPTURE-corrected images. Some small lesions (<1 cm in size) that may have been missed in the uncorrected images due to motion blurring can be better depicted in CAPTURE-corrected images. Finally, Figure 5 illustrates that, according to the blind review conducted by the radiologists, CAPTURE significantly improved the image quality in all cases (p<0.05).
Discussion
CAPTURE makes no assumptions about the MRI signal except that respiration frequencies are below 1 Hz. Therefore, pre-processing steps like baseline correction, detrending and principal component analysis (PCA) which may distort the true physiological content are not necessary. Furthermore, the navigators of CAPTURE can be flexibly placed along any direction regardless of the imaging orientation. More than one navigator along different directions can also be used for detecting two or more different types of motion.Conclusion
CAPTURE provides a robust and fully-automated solution for obtaining 4D motion-resolved images in a free-breathing setting. With its unique tuning feature, CAPTURE can adapt to large inter-subject and inter-scan variations. CAPTURE also enables better lesion delineation due to improved image sharpness, increasing the detectability of small lesions.Acknowledgements
No acknowledgement found.References
1. Lin W, Guo J, Rosen MA, et al. Respiratory motion-compensated radial dynamic contrast enhanced (DCE)-MRI of chest and abdominal lesions. Magn. Reson. Med. 2008;60(5):1135– 1146.
2. Grimm R, Fürst S, Souvatzoglou M, et al. Self-gated MRI motion modeling for respiratory motion compensation in integrated PET/MRI. Med. Image Anal. 2015;19(1):110–120.
3. Buerger C, Clough RE, King AP, et al. Nonrigid Motion Modeling of the Liver From 3-D Undersampled Self-Gated Golden-Radial Phase Encoded MRI. IEEE Trans. Med. Imaging. 2012;31(3):805–815.
4. Feng L, Axel L, Chandarana H, et al. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn. Reson. Med. 2016;75(2):775–788.
5. Campbell-Washburn AE, Xue H, Lederman RJ, et al. Real-time distortion correction of spiral and echo planar images using the gradient system impulse response function. Magn. Reson. Med. 2016;75(6):2278–2285.
6. Paul J, Divkovic E, Wundrak S, et al. High-resolution respiratory self-gated golden angle cardiac MRI: Comparison of self-gating methods in combination with k-t SPARSE SENSE. Magn. Reson. Med. 2015;73(1):292–298.
Figures