0478
Joint PET-MR image registration for cardiac and respiratory motion correction of simultaneous cardiac PET-MR1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare, Frimley, United Kingdom, 4MR R&D Collaborations, Siemens Medical Solutions, New York, NY, United States, 5Biomedical Image Analysis Group, Department of Computing, Imperial College London, London, United Kingdom
Synopsis
Cardiac Positron Emission Tomography (PET) can provide diagnostic information about myocardial perfusion and metabolism with excellent sensitivity. The main challenge is physiological motion of the heart due to breathing and heartbeat. Here we present a joint PET-MR image registration approach which provides non-rigid cardiac and respiratory motion information utilising image data from a simultaneous PET-MR scan of less than 4min. In addition, attenuation correction information is also derived from the MR scan. The motion information is utilised in a motion-corrected PET image reconstruction which improves PET image quality, enhances visualisation of small features and increases measured uptake values by 22±7%.
INTRODUCTION
Cardiac Positron Emission Tomography (PET) can provide diagnostic information about myocardial perfusion and metabolism with excellent sensitivity. The main challenge is physiological motion of the heart due to breathing and heartbeat. Several in-vivo studies have shown that in simultaneous PET-MR scans, MR can be used to determine the motion of the heart which is then used to correct for motion in PET images1–6. Here we propose an approach which utilises both MR and PET image data in a joint motion estimation to obtain 3D non-rigid respiratory and cardiac motion information (motion fields). This approach provides more accurate motion fields than motion estimation using only MR or only PET images. The motion fields are then utilised in a motion-corrected PET image reconstruction to improve image quality.METHODS
Patient population: Three patients (49±11years, 80±8kg, all male) were imaged 175±19min after 18F-FDG injection (334±17MBq).
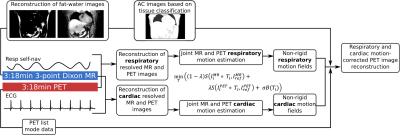
Free-breathing data acquisition: Simultaneous PET-MR data was obtained during a 3:18min free-breathing scan. MR data acquisition was carried out with a triple-echo prototype Dixon-based GRE Golden angle Radial Phase Encoding (GRPE) sequence (TE = 1.2/2.7/4.2ms, FA = 10°) allowing for the reconstruction of attenuation correction (AC) maps and dynamic motion-resolved 3D image data7. Spatial resolution of MR was 1.9x3.2x3.2mm3 and 2.1x2.1x2mm3 for PET with a FOV covering the entire thorax including the arms.
Reconstruction of respiratory and cardiac motion resolved MR and PET images: The MR and PET data were first binned into 8 respiratory motion states based on a 1D MR self-navigator signal7. In a second step MR and PET data were separated into 8 cardiac motion states based on an external ECG. 4D motion-resolved MR images were reconstructed offline using a non-Cartesian iterative SENSE approach with temporal and spatial regularization8. 4D motion-resolved PET images were reconstructed using STIR (3D OSEM, 23 subsets, 3 iterations, 4 mm isotropic Gaussian post-filtering)9.
Respiratory and cardiac motion estimation: 3D non-rigid respiratory and cardiac motion was estimated using a spline-based registration algorithm (MIRTK10) by minimizing the following function:
$$\min_T \big( ( 1-\lambda ) S ( I_i^{MR} \odot T_i, I_{ref}^{MR}) + \lambda S ( I_i^{PET} \odot T_i, I_{ref}^{PET} ) + \sigma B(T_i) \big)$$
where S is the image similarity metric (normalised mutual information), Ii are the 3D images at different motion states i, Ti is a 3D non-rigid transformation for a certain motion state and B is a regularisation term of the spline interpolation. The weighting between MR and PET image information is determined by λ. Combining both respiratory and cardiac motion information provided 8 respiratory x 8 cardiac = 64 different motion fields. The reference motion states ref were end-expiration for respiratory and mid-diastole for cardiac motion.
AC images: From the three-point Dixon MR data fat and water images were reconstructed and separated into six different tissue types11. Literature-based attenuation values were assigned to the different tissue types to obtain AC images.
Motion-corrected PET image reconstruction (PET-MCIR): Respiratory and cardiac motion fields were utilised in PET-MCIR to compensate for both types of physiological motion. The AC images were transformed to the different motion states to ensure accurate quantification. The reconstruction was carried out with STIR (3D OSEM, 23 subsets, 3 iterations, 4 mm isotropic Gaussian post-filtering).
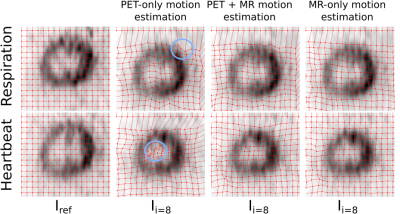
Evaluation: We reconstructed PET images from motion fields with λ = 0.5 (joint PET-MR) and compared them to λ = 0 (PET-only) and λ = 1 (MR-only).
RESULTS
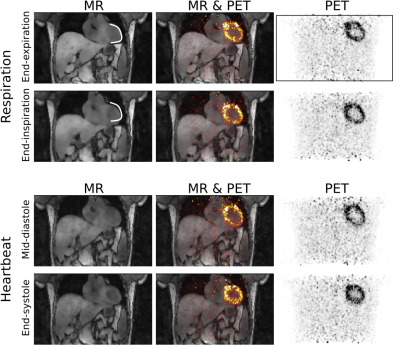
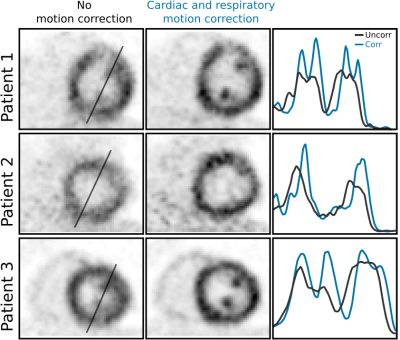
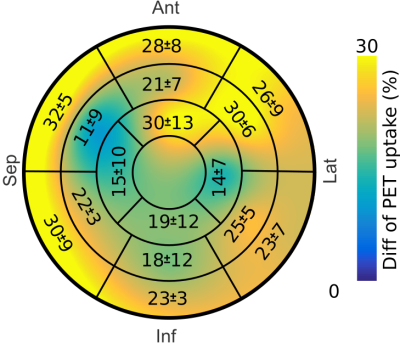
Fig. 2 shows motion-resolved MR and PET images and motion fields. PET-only image registration suffered from distortions due to high noise in the motion-resolved PET images. MR-only image registration provided accurate respiratory information but could not capture cardiac motion reliably due to the poor contrast between myocardium and blood in the GRE images (Fig. 3). The proposed joint PET-MR image registration overcame those challenges, and provided accurate 3D non-rigid respiratory and cardiac motion information. PET-MCIR utilising this motion information, minimized motion blurring, increased measured uptake values and improved visualisation of small structures, such as papillary muscles (Fig. 4). An average increase in measured uptake values across the entire myocardium of 22±7% was achieved with MCIR (Fig. 5).CONCLUSION
The proposed joint PET-MR image registration provided accurate cardiac and respiratory motion information, strongly improving PET image quality and PET quantification. The accuracy of the motion estimation also depends on PET uptake and future work will focus on assessing this approach in patients with myocardial defects.Acknowledgements
No acknowledgement found.References
1. Würslin C, Schmidt H, Martirosian P, et al. Respiratory motion correction in oncologic PET using T1-weighted MR imaging on a simultaneous whole-body PET/MR system. J Nucl Med. 2013;54(3):464–471.
2. Fayad H, Odille F, Schmidt H, et al. The use of a generalized reconstruction by inversion of coupled systems (GRICS) approach for generic respiratory motion correction in PET/MR imaging. Phys Med Biol. 2015;60(6):2529–2546.
3. Furst S, Grimm R, Hong I, et al. Motion Correction Strategies for Integrated PET/MR. J Nucl Med. 2015;56(2):261–269.
4. Rank CM, Heußer T, Wetscherek A, et al. Respiratory motion compensation for simultaneous PET/MR based on highly undersampled MR data. Med Phys. 2016;43(12):6234–6245.
5. Petibon Y, Huang C, Ouyang J, et al. Relative role of motion and PSF compensation in whole-body oncologic PET-MR imaging. Med Phys. 2014;41(4):42503.
6. Kolbitsch C, Ahlman MA, Davies-Venn C, et al. Cardiac and Respiratory Motion Correction for Simultaneous Cardiac PET/MR. J Nucl Med. 2017;58(5):846–852.
7. Kolbitsch C, Neji R, Fenchel M, Mallia A, Marsden P, Schaeffter T. Fully integrated 3D high-resolution multicontrast abdominal PET-MR with high scan efficiency. Magn Reson Med. Mei 2017.
8. Cruz G, Atkinson D, Buerger C, Schaeffter T, Prieto C. Accelerated motion corrected three-dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magn Reson Med. 2016;75(4):1484–1498.
9. Thielemans K, Tsoumpas C, Mustafovic S, et al. STIR: software for tomographic image reconstruction release 2. Phys Med Biol. 2012;57(4):867–883.
10. Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18(8):712–721.
11. Martinez-Moller A, Souvatzoglou M, Delso G, et al. Tissue Classification as a Potential Approach for Attenuation Correction in Whole-Body PET/MRI: Evaluation with PET/CT Data. J Nucl Med. 2009;50(4):520–526.
Figures