0477
Cardiac and respiratory motion-corrected whole-heart PET-MR imaging for simultaneous assessment of coronary anatomy, cardiac function and myocardial integrity1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare, Frimley, United Kingdom, 3Nuclear Medicine, TU Munich, Munich, Germany
Synopsis
Image degradation due to cardiac and respiratory motion remains a challenge for cardiac PET-MR imaging. Here we propose a simultaneous dual-phase PET and Coronary MR angiography (CMRA) acquisition and reconstruction framework that allows for the visualisation of coronary anatomy, estimation of ventricular function and motion-corrected myocardial PET in a single efficient examination. A validation study in healthy subjects shows that left ventricular ejection fraction can be estimated from dual-phase CMRA images at 3T. Results from patients with cardiovascular disease show improvements in respiratory motion corrected dual-phase CMRA and in PET image quality when applying both cardiac and respiratory motion correction.
Introduction
Simultaneous PET-MR is a promising technique for whole-heart cardiac imaging. However, data acquisition for both PET and MR is slower than the physiological motion of the heart, inducing blurring in both PET and MR images, and artefacts due to mismatches between the attenuation maps and emission data in PET images. Recent developments for cardiac PET-MR have focused on alleviating this problem by using motion estimated from MR images to correct the PET data. However, lack of preparatory pulses and/or sufficient resolution prevents the use of those MR images for diagnostic purposes1,2. In this work we propose a motion compensated whole-heart PET-MR acquisition and reconstruction scheme based on a dual-phase Coronary MR Angiography (CMRA) sequence that provides: 1) coronary anatomy visualization from CMRA acquisition, 2) left ventricular ejection fraction estimation from dual-phase anatomic MR acquisition3, and 3) quantification of myocardial integrity from PET data, in a single efficient examination.Methods
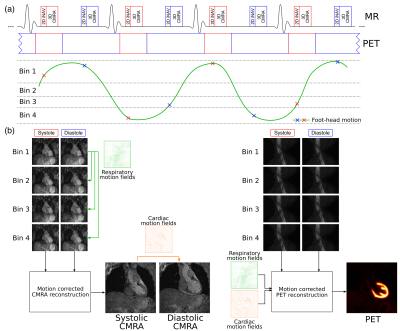
An ECG-triggered free-breathing dual-phase CMRA sequence is acquired simultaneously with list-mode cardiac PET data (Fig1a). CMRA data is acquired with a 3D T1-weighted spoiled gradient echo sequence, with a fully sampled golden-step Cartesian with spiral profile order sampling trajectory4. 2D image navigators5 are acquired before the CMRA data in each heartbeat. Non-rigid respiratory and cardiac motion is estimated from MR images, and the MR-derived deformation fields are used to correct for both the CMRA (respiratory motion correction for each cardiac phase6) and the simultaneously acquired PET data (respiratory and cardiac motion correction) (Fig1b).
Six healthy subjects were scanned on a Biograph mMR 3T scanner (Siemens Healthcare, Germany) using a prototype implementation of the proposed gradient echo dual-phase CMRA sequence (resolution = 1x1x2mm, FOV = 304x304x80-88mm, coronal orientation, TR/TE = 3.7/1.7ms, FA=12°, T2prep duration = 50ms). Two subject-specific trigger delays were defined coinciding with the systolic and diastolic rest periods and an acquisition window ranging from 89 to 104ms was used. Additionally, a conventional multi-slice and multi-breath-hold 2D short-axis cine acquisition was performed to validate the estimation of left ventricular function. The whole PET-CMRA framework was tested in three patients with documented ischemic heart disease, using the same acquisition parameters described for the healthy subjects, with 18F-FDG list-mode PET data acquired simultaneously during the whole dual-phase CMRA acquisition. In patients acquisition was performed ~1-2 min after injection of a Gd-based contrast agent.
Dual-phase CMRA and PET data were reconstructed without motion correction (NMC) and with the proposed motion correction scheme (MC). PET images were also reconstructed with respiratory motion correction only (RespMC) for comparison purposes. PET image reconstruction was performed offline using a reconstruct-transform-average7 motion correction in Siemens e7 Tools (OSEM, 3 iterations, 21 subsets, PSF modelling, 2.03x2.08x2.08mm voxel size, 127x344x344 matrix size).
Results
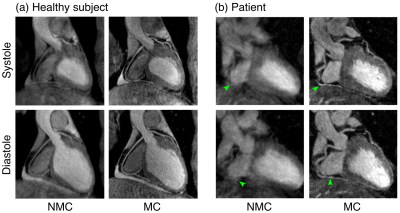
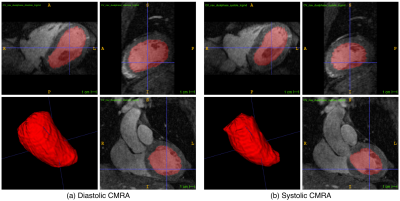
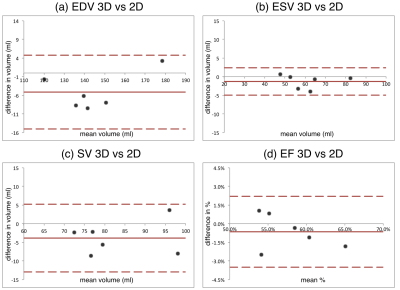
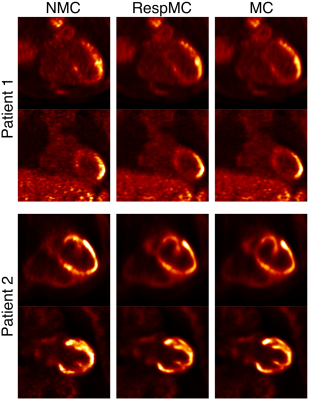
Systolic and diastolic CMRA images were reformatted to visualize the left anterior descending (LAD) and right coronary artery (RCA) simultaneously. MC improves the visualisation of the coronary arteries for healthy subjects and patients (Fig 2), increasing the visible length and sharpness of the vessels in both systole and diastole. Good contrast between blood-pool and myocardium allows for left ventricular volumetric quantification, as shown in Fig 3. End-diastolic and end-systolic volumes (EDV and ESV, respectively) estimated from dual-phase data show results comparable to the multi-slice multi-breath-hold 2D cine images, with an average underestimation of 5.1ml and 1.3ml respectively (Fig 4a-b). Functional indices including stroke volume (SV) and ejection fraction (EF) result therefore in good agreement with the conventional approach, with an average bias of -3.8ml and -0.65% respectively (Fig 4c-d). Applying respiratory motion correction (RespMC) to the PET images reduced levels of noise and resulted in sharper myocardial uptake compared to uncorrected PET images (Fig 5). Inclusion of cardiac motion correction produced further improvements compared to RespMC images.Conclusion
Here we present a whole-heart cardiac PET-CMRA acquisition and reconstruction scheme that allows the simultaneous visualisation of coronary anatomy, quantification of ventricular volumes and ejection fraction, and motion-corrected myocardial PET in a 3T PET-MR scanner. Respiratory and cardiac motion is estimated from MR images, and used to correct both the CMRA and PET data producing diagnostic images for both modalities. This approach has the potential for allowing comprehensive non-invasive assessment of ischemic heart disease in a single and time-efficient examination.Acknowledgements
This work is funded by the King’s College London & Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1) and EPSRC Grant EP/N009258/1.References
(1) Kustner T et al. MR-based respiratory and cardiac motion correction for PET imaging, Med Imag Analysis 2017;42: 129-144
(2) Kolbitsch C et al. Cardiac and respiratory motion correction for simultaneous cardiac PET-MR. J Nucl Med 2017;58. doi: 10.2967/jnumed.115.171728
(3) Uribe S et al. Volumetric cardiac quantification by using 3D dual-phase whole-heart MR imaging. Radiology 2008;248:606–614
(4) Prieto C et al. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J Magn Reson Imaging 2015;41:738–746
(5) Henningsson M et al. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn Reson Med 2012;67:437–445
(6) Munoz C et al. Motion-corrected simultaneous cardiac positron emission tomography and coronary MR angiography with high acquisition efficiency. Magn Reson Med 2017. doi: 10.1002/mrm.26690
(7) Picard Y et al. Motion correction of PET images using multiple acquisition frames. IEEE Trans Med Imaging 1997;16:137–144
Figures