0470
Probing microstructural heterogeneity and mild traumatic brain injury-induced gray matter alterations in the rat brain using diffusion kurtosis imaging1Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2University of Eastern Finland, Kuopio, Finland
Synopsis
In heterogeneous brain tissue microenvironments, the probability distribution of spin displacements deviates from Gaussianity. Examining the non-Gaussian behavior of the diffusion-encoded signal with diffusion kurtosis imaging (DKI) could potentially allow probing cellular heterogeneity and injury-induced changes that are not discernible in the limited (q,t)-regime that pulsed-gradient DTI with conventional b-values explores. Here, we investigated the sensitivity of DKI to intrinsic cellular heterogeneity and mild traumatic brain injury (mTBI)-induced gray matter (GM) alterations in the rat brain. The results demonstrate distinct contrasts in mean kurtosis maps reflecting microstructural heterogeneity in GM regions, and detection of region-specific alterations in the cortex and thalamus.
Purpose
Under the effects of restrictions posed by local tissue microenvironment, the probability distribution of spin displacements in the brain deviates from Gaussianity. The non-Gaussian behavior of the diffusion NMR signal at high b-values encodes potential microstructural information that can be extracted by examining higher-order statistics of random molecular motion, e.g., the kurtosis1,2, which may not be probed with the rank-2 diffusion tensor model. Diffusion kurtosis imaging (DKI) could thus potentially reveal sensitivity to detect cytoarchitectural heterogeneity and subtle injury-induced alterations in the brain, particularly in gray matter (GM) regions, that are not discernible in the limited (q,t) regime that pulsed-gradient DTI with conventional b-values explores3. Here, we investigated the sensitivity of DKI to intrinsic cellular heterogeneity in GM regions of the rat brain, and to detect cellular-scale alterations following mild traumatic brain injury (mTBI).Methods
Adult Wistar rats (n=5) were subjected to mTBI via the lateral fluid percussion (LFP) model. Five weeks after induction of mTBI, brains of rats with LFP (n=5) and age-matched controls (n=5) were perfused with 4% PFA. Imaging experiments were performed on an 11.7T Bruker scanner (100 G/cm single-axis gradients) using a 20-mm birdcage transceiver coil. Multi-shell diffusion MRI (dMRI) data were acquired using a 3D diffusion-weighted gradient-and-spin-echo (DW-GRASE) sequence4 with twin-navigator echoes and the following parameters: rare-factor/EPI factor=4/3, TR/TE=700/33 ms, δ/Δ=3/10 ms, 2 averages, and isotropic resolution=200-µm. For DKI, 2 non-diffusion-weighted images and 30 uniformly-distributed diffusion directions5 were acquired over two b-value shells (b=3000 and 6000 s/mm2). To measure signal attenuation curves, data from one brain were additionally acquired with 4 b-value shells of 1500, 3000, 4500, and 6000 s/mm2. Mean kurtosis (MK) maps were calculated from kurtosis-tensor reconstruction using constrained linear-weighted DKI fitting6, with no spatial smoothing. DTI reconstruction was done using data acquired with b=3000 s/mm2. Images from each rat were co-registered to one control brain as the reference using diffeomorphic registration. After MRI, nuclei-specific DAPI staining was used to compare cellular heterogeneity in select brain regions.Results
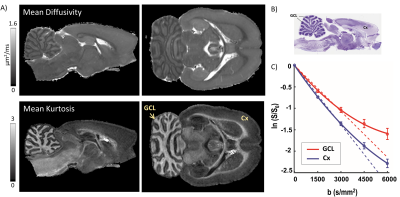
Fig. 1 shows mean diffusivity (MD) and MK maps of a representative control rat brain. MK maps revealed distinctly heterogeneous contrast in GM regions of the rat brain. Fig. 1A shows striking MK contrast in the cerebellar cortex, which has a highly organized cytoarchitecture. The granule cell layer (GCL) of the cerebellum was marked by significantly (p<0.001) elevated kurtosis (0.92±0.04) compared to the molecular layer (0.44±0.03). The GCL consists of very small densely-packed granule cells (Nissl-stained section in Fig. 1B), and appeared significantly enhanced with MK values comparable to most white matter regions (MK maps in Fig 1). In comparison, the cerebral cortex (Cx) exhibited relatively low MK (0.50±0.03) reproducibly across all control rats. Plots of log-signal attenuation versus b-value in the GCL and Cx (Fig. 1C) reveal a clear deviation from Gaussianity at b-values >3000 s/mm2, with markedly high kurtosis in the GCL.
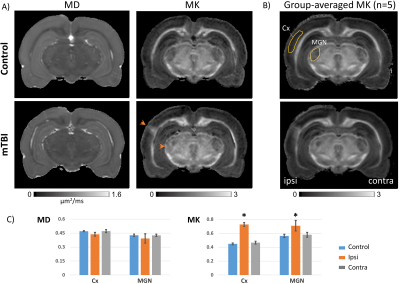
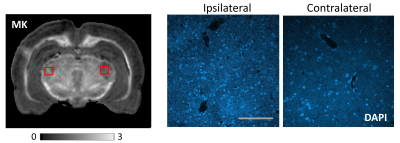
Fig. 2 shows comparison of MD and MK maps of control and LFP-injured rat brains. Compared to control rats, the LFP-injured brains revealed a sharp increase in MK localized to selective GM regions of the ipsilateral cortex (Cx) and medial geniculate nucleus of the thalamus (MGN) (arrowheads, Fig. 2A). In comparison, no significant differences were seen in MD maps (Fig. 2A). Group-averaged MK maps of control and LFP-injured rats (n=5 each) show reproducible and selective enhancement of the Cx and MGN after mTBI, with clear ipsilateral and contralateral differences in the two GM regions (Fig. 2B). Plots of MD and MK measurements (Fig. 2C) demonstrate significantly (p<0.005) elevated MK in the ipsilateral Cx and MGN of LFP-injured brains compared to the contralateral regions and control brains, with no significant differences in MD. Comparison with histopathological assessment using DAPI-staining revealed localized gliosis in the ipsilateral MGN and Cx with significantly increased number of cell-nuclei, compared to the contralateral side (DAPI-stained sections in Fig. 3).
Discussion and Conclusion
The results reveal unique sensitivity of diffusional kurtosis to probe cytoarchitectural heterogeneity and subtle gray matter cellular alterations in the rat brain. Interestingly, selective enhancement of the GCL in kurtosis maps (Fig. 1) is strikingly similar to the selective enhancement of this cerebellar layer observed in ADC maps at ultra-short diffusion times with oscillating-gradient spin-echo (OGSE) sequences7. These results show that non-Gaussian behavior of the diffusion signal at high b-values can effectively allow probing the effects of sub-voxel restrictions over short length scales even at long diffusion times accessible with pulsed-gradient (PGSE) sequences. Finally, the sensitivity of diffusional kurtosis to detect subtle and localized cellular changes in the Cx and MGN even in the absence of lesions will provide important insights into region-specific pathological processes following mTBI8,9, which are not detectable with conventional DTI in the low diffusion-weighting regime.Acknowledgements
This work was supported by the National Institutes of Health (NIH grants R21NS096249 and R03EB017806) and Academy of Finland.References
1. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005;53(6):1432-1440.
2. Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 2010;23(7):698-710.
3. Wu EX, Cheung MM. MR diffusion kurtosis imaging for neural tissue characterization. NMR Biomed 2010;23(7):836-848.
4. Aggarwal M, Mori S, Shimogori T, Blackshaw S, Zhang J. Three-dimensional diffusion tensor microimaging for anatomical characterization of the mouse brain. Magn Reson Med 2010;64(1):249-261.
5. Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 1999;42(3):515-525.
6. Tabesh A, Jensen JH, Ardekani BA, Helpern JA. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med 2011;65(3):823-836.
7. Aggarwal M, Jones MV, Calabresi PA, Mori S, Zhang J. Probing mouse brain microstructure using oscillating gradient diffusion MRI. Magn Reson Med 2012;67(1):98-109.
8. Grossman EJ, Ge Y, Jensen JH, Babb JS, Miles L, Reaume J, Silver JM, Grossman RI, Inglese M. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J Neurotrauma 2012;29(13):2318-2327.
9. Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, Gullapalli RP. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage 2012;59(1):467-477.
Figures