0457
Cortical GABA levels correlate with visual search performance in children with autism spectrum disorder1School of Health Sciences, Purdue University, West Lafayette, IN, United States, 2Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, United States, 3Department of Speech, Language, & Hearing Sciences, Purdue University, West Lafayette, IN, United States
Synopsis
Although diagnosed based on sociocommunicative deficits, autism spectrum disorder (ASD) is characterized by superior performance on selective attention tasks, particularly visual search. In neurotypical individuals, region-specific concentrations of GABA are associated with differences in attention and perception. While it has been hypothesized that ASD may be associated with an excitatory-inhibitory imbalance, it remains unclear how this may contribute to autistic search advantage. To test this, 10 children with ASD participated in a magnetic resonance spectroscopy (MRS) study using MEGA-semi-LASER to detect GABA concentrations in target regions, including the frontal eye fields, temporal parietal junction, and visual cortex.
Introduction
Children, adolescents, and adults diagnosed with autism spectrum disorder (ASD) excel at visual search compared to their typically developing peers1. Evidence of augmented ASD search superiority with increasing target-distractor similarity has been used to support the hypothesis that enhanced discrimination contributes to faster search. Others have suggested that faster search may be due to differences in the distribution of attention rather than enhanced lower-level perceptual processing. Thus, the precise mechanism underlying superior search and the brain bases for this advantage remains unknown. One model has hypothesized that ASD may result from an imbalance of glutamatergic and GABAergic signaling2. In addition, inter-individual differences in attention and perception in neurotypical individuals are associated with region-specific concentrations of GABA3,4. However, the contributions of abnormal GABAergic function to autistic superior search abilities has not been determined. To test this, we used magnetic resonance spectroscopy (MRS) to measure GABA using MEGA-semi-LASER in the visual cortex, temporal parietal junction, and frontal eye fields.Methods
Ten children with ASD (8 males, 2 females, Average Age = 12 years, Range = 10-14 years) participated in the current study. Participants completed multiple experimental paradigms prior to scanning including a visual search task (VST)5. In the VST, the participant's task was to indicate the presence or absence of a target (vertical line) embedded within arrays of distractors (tilted lines) that varied in set size (18, 24, 36 items) (Figure 1). Search speed was measured as reaction time (RT) to determine presence or absence of the target item. The slopes (a measure of search efficiency, reflecting the RT cost of each additional distractor) and y-intercepts (associated with non-search, perceptual processes) of the RT x set size functions were calculated for target-present and target-absent conditions. All scans were obtained on a 3T Siemens Prisma MR Scanner (VE11B) at Purdue University. MEGA-semi-LASER6,7 (TE = 68ms, TR = 2000ms, Averages = 128, CMRR) was used to measure GABA and tCr (Cr + PCr) in the bilateral visual cortex (VIS, voxel size = 30mm x 30mm x 20mm), right temporal parietal junction (TPJ, voxel size = 30mm x 30mm x 20mm), and right frontal eye fields (FEF, voxel size = 30mm x 30mm x 20mm)3 (Figure 2). MEGA-semi-LASER, rather than MEGA-PRESS, was chosen to allow us to fit voxels closer to the skull due to the smaller head size in children compared to adults, thus maximizing grey matter contribution in the cortical voxels. Difference and Off spectra were quantified using LCModel8. One VIS spectrum was excluded due to poor spectrum quality. Spearman correlation tests and Spearman partial correlation tests were used to assess relationships between GABA levels and VST parameters (RT, y-intercept, and slope).Results
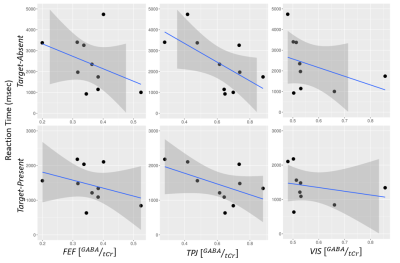
After controlling for Non-Verbal IQ (NVIQ), VIS GABA/tCr levels were negatively correlated with RT in both target-present (ρ = -0.844, p = 0.0001) and target-absent (ρ = -0.744, p = 0.006) conditions (Figure 3). In addition, y-intercept for target-present trials (ρ = -0.657, p = 0.033) and y-intercept (ρ = -0.611, p = 0.059) and slope (ρ = -0.685, p = 0.021) for target-absent trials were negatively correlated with VIS GABA levels. After controlling for NVIQ, TPJ GABA/tCr levels were negatively correlated with target-present (ρ = -0.769, p = 0.0015) and target-absent (ρ = -0.779, p = 0.001) RT. TPJ GABA/tCr levels were also negatively correlated with target-absent y-intercept (ρ = -0.559, p = 0.075) and slope (ρ = -0.627, p = 0.033). No significant relationships were found between FEF GABA/tCr and VST parameters. All results are shown in Table 1.Discussion
Preliminary results suggest that greater GABA levels in VIS are associated with faster search, greater search efficiency (i.e., reduced slope), and faster non-search-related perceptual processes (i.e., lower y-intercepts), potentially indicating that enhanced discrimination contributes to faster, more efficient search in ASD. Our results are consistent with Edden et al (2009), who showed that greater GABA levels in visual cortex was associated with increased discrimination4. Additionally, higher GABA concentrations in the TPJ were associated with enhanced search-related indices. However, a lack of associations between FEF GABA concentrations and VST measures suggests that ASD search advantage may be related to enhanced discrimination rather than attentional filtering account.Conclusion
Our preliminary findings suggest that better visual search performance may be related to increased GABA in the visual cortex and temporal parietal junction, but not the frontal eye fields in children with autism spectrum disorder (ASD). Data collection is currently ongoing; future analyses will compare current results with performance and MRS data collected from a cohort of age- and IQ-matched typically developing comparison children. Nonetheless, this study demonstrates that GABA concentrations are related to a domain of enhanced performance in children diagnosed with ASD.Acknowledgements
This study was funded by a Purdue Institute for Integrative Neuroscience pilot grant. We would also like to acknowledge Dr. James Murdoch for his help generating MEGA-Semi-LASER basis sets for LCModel.References
1. Kaldy Z, Giserman I, Carter AS, Blaser E. The Mechanisms Underlying the ASD Advantage in Visual Search. J Autism Dev Disord. 2016;46(5):1513-1527. doi:10.1007/s10803-013-1957-x.
2. Hussman JP. Suppressed GABAergic Inhibition as a Common Factor in Suspected Etiologies of Autism. J Autism Dev Disord. 2001;31(2):247-248. doi:10.1016/0002-9610(92)90118-B.
3. Sumner P, Edden RAE, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13(7):825-827. doi:10.1038/nn.2559.
4. Edden RAE, Muthukumaraswamy SD, Freeman TCA, Singh KD. Orientation Discrimination Performance Is Predicted by GABA Concentration and Gamma Oscillation Frequency in Human Primary Visual Cortex. J Neurosci. 2009;29(50):15721-15726. doi:10.1523/JNEUROSCI.4426-09.2009.
5. Kemner C, Van Ewijk L, Van Engeland H, Hooge I. Brief report: Eye movements during visual search tasks indicate enhanced stimulus discriminability in subjects with PDD. J Autism Dev Disord. 2008;38(3):553-558. doi:10.1007/s10803-007-0406-0.
6. Andreychenko A, Boer VO, Arteaga De Castro CS, Luijten PR, Klomp DWJ. Efficient spectral editing at 7 T: GABA detection with MEGA-sLASER. Magn Reson Med. 2012;68(4):1018-1025. doi:10.1002/mrm.24131.
7. Marjańska M, Auerbach EJ, Valabrègue R, Van de Moortele P-F, Adriany G, Garwood M. Localized 1H NMR spectroscopy in different regions of human brain in vivo at 7 T: T2 relaxation times and concentrations of cerebral metabolites. NMR Biomed. 2012;25(2):332-339. doi:10.1002/nbm.1754.
8. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672-679. doi:10.1002/mrm.1910300604.
Figures