0453
Real time observation of shifts in cerebral metabolism caused by cocaine administration via MRS, DNP, and NMR1Box 100245, University of Florida, Gainesville, FL, United States, 2University of Florida, Gainesville, FL, United States, 3University of Colorado, Boulder, CO, United States
Synopsis
The high energy requirements of the brain are sustained by a unique metabolic relationship between astrocytes and neurons. Here, we show how cocaine administration shifts neurometabolism at a fundamental level. Using a novel approach combining dynamic nuclear polarization-enabled metabolic flux measurements with steady state magnetic resonance measures of metabolite pools, we reveal acute cocaine administration disrupts the balance of oxidative and non-oxidative metabolic pathways. These results demonstrate significant metabolic shifts in response to cocaine administration, providing insight into the observed short-term effects of cocaine use.
Introduction
A consistently reported finding has been that acute and chronic cocaine administration cause significant alterations in cerebral glucose utilization,1-2 impairs mitochondrial metabolism,3-4 and that these effects are associated with cytotoxicity and autophagic cell death.5-6 Impairments in cellular bioenergetics and suboptimal cellular ‘health’ can in turn adversely impact other neurophysiological processes such as synaptic plasticity and neuronal excitability, efficacy of intracellular signaling cascades, and DAergic and glutamatergic neurotransmission, all of which are reportedly altered by chronic cocaine exposure. While general neurometabolic changes related to glucose utilization following cocaine administration is strongly supported, there is much less understood regarding the in vivo intracellular metabolic pathways that are adversely affected by cocaine or that can contribute to impaired cerebral metabolic activity. It is possible that impaired energy utilization in cocaine subjects arises through glycolytic enzymatic pathway dysfunction and impaired intercellular exchange, which up until now has been difficult to measure in real-time and in situ.Methods
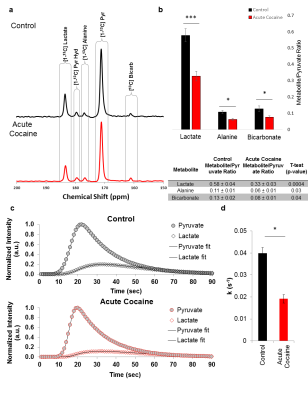
Here, we demonstrate dissolution dynamic nuclear polarization, using 13C-enriched pyruvate, can measure cocaine-induced changes in cerebral pyruvate metabolic flux in vivo. Additionally, we present complementary in vivo and ex vivo 1H and 31P magnetic resonance measurements of cerebral metabolite pools. 13C-enriched pyruvate was polarized at 5 T and <1.2 K prior to dissolution and injection into adult male Long Evans rats in a 4.7 T scanner for 13C flux measurements during the psychoactive period of cocaine (12-13 minutes after injection). 1H and 31P single voxel spectroscopy experiments were similarly performed during the psychoactive period after cocaine injection using an 11.1 T scanner. After voxel spectroscopy, the left cerebral hemispheres were immediately removed, flash frozen, extracted, and polar metabolite levels in the extracts were measured via mass spectrometry and solution state NMR spectrscopy. The right cerebral cortex tissues, corresponding to the sensitive region detected by the 13C coil in the DNP experiment and the 1H coil in the MRS experiments, were flash-frozen and then characterized using high-resolution magic angle spinning (HR-MAS) NMR spectroscopy.Results
Acute administration of a single dose of (10mg/ml/kg) cocaine significantly suppresses the conversion of pyruvate to lactate as observed by dissolution DNP. Additionally, the rate of pyruvate to lactate conversion is significantly decreased. Cocaine administration also leads to significant increases in circulating glutamate and aspartate, while glutamine and lactate levels are decreased. The combined measures of metabolic flux and metabolite pools enable us to determine how cocaine administration changes energy utilization within the brain to drive the production of glutamate and aspartate. These findings illustrate how in vivo metabolic flux measurements can probe the health of and synergy between neurons and astrocytes. The approach we demonstrate can be combined with more traditional, correlative MRI approaches to understanding brain development, function, cognition, and neurodegeneration to enable the identification of early metabolic markers for specific neurodegenerative diseases as well as provide a more fundamental understanding of brain function and health at the molecular and cellular levels.Acknowledgements
All magnetic resonance experiments were performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and the State of Florida.References
1. Volkow, N. D. et al. Long-term frontal brain metabolic changes in cocaine abusers. Synapse 11, 184-190 (1992).
2. Volkow, N. D. et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry 148, 621-626 (1991).
3. Zhou, Z., Yuan, Q., Mash, D. C. & Goldman, D. Substance-specific and shared transcription and epigenetic changes in the human hippocampus chronically exposed to cocaine and alcohol. Proc Natl Acad Sci U S A 108, 6626-6631 (2011).
4. Li, Y. et al. 1H NMR-based metabonomics in brain nucleus accumbens and striatum following repeated cocaine treatment in rats. Neuroscience 218, 196-205 (2012).
5. Lehrmann, E. et al. Transcriptional profiling in the human prefrontal cortex: evidence for two activational states associated with cocaine abuse. Pharmacogenomics J 3, 27-40 (2003).
6. Badisa, R. B. et al. N-acetyl cysteine mitigates the acute effects of cocaine-induced toxicity in astroglia-like cells. PLoS One 10, e0114285 (2015).
Figures