0452
IMPORTANCE OF THE LACTATE SHUTTLE FOR BRAIN ACTIVATION: AN IN VIVO LOCALIZED 1H-MRS AND FUNCTIONAL MRI STUDY DURING WHISKER STIMULATION1CNRS/Université Bordeaux, Centre de Résonance Magnétique des Systèmes Biologiques UMR 5536, Bordeaux, France, Metropolitan, 2CH Lausanne, Switzerland, Département de Physiologie, Lausanne, Switzerland, 3Lausanne University Hospital, Department of Clinical Neurosciences, Laboratory of Cellular and Molecular Neurotherapies (LCMN), Lausanne, Switzerland
Synopsis
Although several in vitro and ex vivo evidence support the existence of lactate exchange between astrocytes and neurons, a direct demonstration in vivo is still lacking.
The aim of this study was to determine if the neuronal lactate transporter MCT2 is required for proper substrate use by neurons during brain activation. We therefore quantified the brain lactate content by 1H-NMR spectroscopy shRNA-control injected rats (called UNIV rats), MCT4 knockdown rats (called MCT4 rats) and MCT2 knockdown rats (called MCT2 rats), at rest or during whisker stimulation. Moreover, we examined the BOLD fMRI response of the somatosensory cortex associated with whisker stimulation.
INTRODUCTION
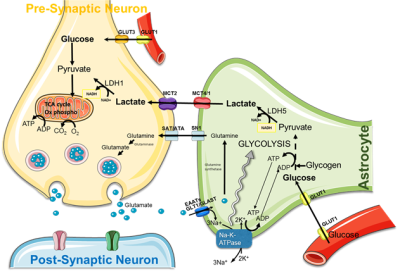
The brain possesses intrinsic mechanisms that allow for its major substrate, i.e. glucose, the regulation of both its contribution and its utilization depending on local cerebral activity variations. Despite the general recognition of this principle, the cellular and molecular mechanisms that underlie such a tight relationship between neuronal activity and energetic metabolism are still unknown and highly debated. More than 100 years ago, Camillo Golgi suggested, at the sight of their cyto-architectural connexions with endothelial cells and neurons, that astrocytes could play a central role in the distribution of energetic substrates between blood vessels and neurons. During the last decade, series of studies have been performed, both in vitro and ex vivo, and they showed that lactate, produced by astrocytes, could be an efficient energetic substrate for neurons, supporting the hypothesis of a lactate shuttle between astrocytes and neurons. However, this shuttle, called ANLS (astrocyte-neuron lactate shuttle) (Fig.1) has never been demonstrated in vivo.
Using in vivo 1H nuclear magnetic resonance (NMR) spectroscopy and genetically-modified rats (modified for a specific monocarboxylate transporter or MCT, a lactate transporter, key partners in this shuttle), our aim is to prove the existence of a lactate transfer from astrocytes to neurons during brain activation (obtained by whisker activation directly into the magnet).
METHODS
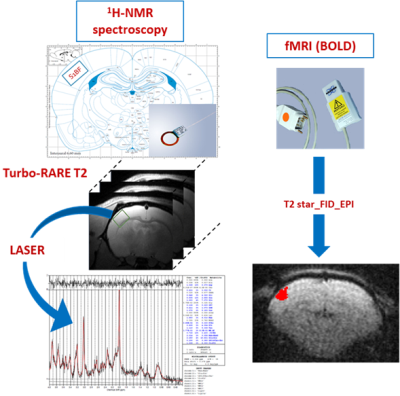
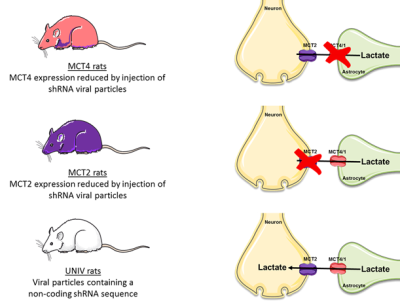
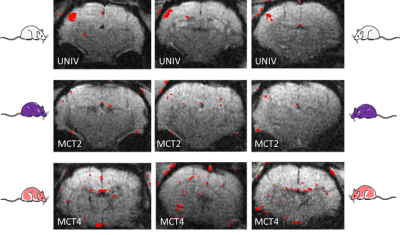
The innovative point is that this study was performed not only on control rats but also on rats that were down regulated either for the neuronal lactate transporter (MCT2) or for the astrocytic one (MCT4) (Fig.3). 3 groups of adults Wistar males were used: Control rats (called UNIV rats, n=17), rats that previously received a local injection into the somatosensory cortex of a adenoviral vector encoding a shRNA directed against MCT2 (called MCT2 rats, n=17) or a shRNA directed against MCT4 (called MCT4 rats, n=8). Animals were slightly anaesthetized using medetomidine (0.1 ml/100 g body weight). Whisker activation was performed directly into the magnet using an air-pulse system at 8 Hz during the acquisition time. After a T2-weighted sequence, in vivo spectroscopy was performed either at rest (10s) or during whisker activation (20s) using a LASER sequence (TE 19.26 ms, TR 2500 ms, 128 scans, 10 mm MRI Surface coil). The lactate peak at 1.32 ppm was quantified using LCModel (Provencher) after phase and baseline corrections. fMRI was also performed [4] (Fig.2).RESULTS
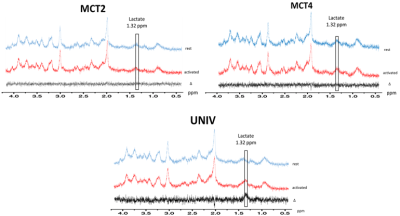
During whisker stimulation, a 20% increase of lactate content was observed between resting and activated states in the barrel cortex of control UNIV rats. This increase was abolished in MCT2 and MCT4 rats (Fig.4). To go further and understand these results, BOLD fMRI was performed. BOLD acquisitions showed that whisker stimulation led to contralateral barrel cortex activation whereas no signal was detected in MCT2 and MCT4 rats (Fig.5).DISCUSSION
This is the first time that a lost in BOLD signal can be evidenced when one of the lactate transporters is suppressed. Since glucose and glucose transporter are still present, these data indicate that each MCT is necessary for the BOLD signal and suggest that if the lactate transfer between astrocytes and neurons is abolished, synaptic activity may be impaired.CONCLUSION
These results highlight the crucial role of astrocytic lactate under brain activation as an important energetic substrate for neurons and shows, for the first time, that the down regulation of key players of the ANLS, the MCTs, leads to the disappearance of the BOLD signal [5]. This strongly suggests that MCTs are necessary for synaptic activity.Acknowledgements
No acknowledgement found.References
[1] Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, et al. (2007) Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55: 1251–1262. pmid:17659524
[2] K.H. Lauritzen, et al.Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolismCereb. Cortex (2013), 10.1093/cercor/bht136
[3] Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, et al. (2014) Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A 111: 12228–12233. pmid:25071212
[4] Adamczak JM, Farr TD, Seehafer JU, Kalthoff D, Hoehn M (2010) High field BOLD response to forepaw stimulation in the mouse. Neuroimage 51: 704–712. pmid:20211267
[5] A neuronal MCT2 knockdown in the rat somatosensory cortex reduces both the NMR lactate signal and the BOLD response during whisker stimulation Leslie Mazuel, Jordy Blanc, Cendrine Repond, Véronique Bouchaud, Gérard Raffard, Nicole Déglon, Gilles Bonvento, Luc Pellerin, Anne-Karine Bouzier-Sore Published: April 7, 2017 https://doi.org/10.1371/journal.pone.0174990
Figures