0449
NMR spectroscopy based blood test to diagnose brain cancer at early stages1Cancer Systems Imaging, University of Texas MD Anderson Cancer Center, Houston, TX, United States, 2Translational Molecular Pathology, University of Texas MD Anderson Cancer Center, Houston, TX, United States, 3Biochemistry and Cell Biology | Chemistry, Rice University, Houston, TX, United States
Synopsis
Early detection of brain cancer will help saving lives. The currently available diagnostic techniques are not robust and expensive. Therefore, it is necessary to develop cost effective, minimally invasive, and highly sensitive analytical tools to identify brain tumors at an early stage. In this study we are investigating metabolism based biomarkers in platelets derived from low grade glioma II, glioblastoma, and healthy patients identified by nuclear magnetic resonance (NMR) spectroscopy. The platelet metabolites - glucose, citrate, and succinate are determined to be promising candidates for identifying and differentiating different stages of brain tumors with implication of employing simple blood based NMR metabolomics for early detection of brain cancer.
Introduction
Platelets are non-nucleated plate like entities circulating in the body and get activated during injuries and help in coagulation of blood. Platelets are not only coagulating agents but also involved in defense mechanism. Many disease prognosis and diagnosis can be correlated with platelet counts1-2. In addition, recent findings have shown that platelets exchange information from diseased or affected parts of the body. The in vitro studies of platelets incubation with GBM cancer cells has confirmed the transfer of RNA’s from the GBM cells to platelets. Also, the study in a set of glioblastoma (GBM) patients have shown the carriage of RNA by the platelets which are specific to GBM3-4. Based on these studies we hypothesized that platelets derived from low grade glioma, high grade glioma and aggressive glioblastoma will have different metabolic profiles. To test our hypothesis, platelets were collected from healthy donors, low-grade glioma with an isocitrate dehydrogenase (IDH) mutation, and high-grade glioblastoma. We have looked at small molecular (<1500 Da) variation in platelets collected from low grade glioma, GBM, and healthy patients employing NMR spectroscopy as an analytical tool.
Methods
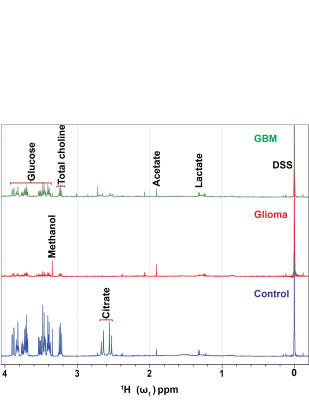
Metabolites were extracted from platelets using a methanol-water mixture, ceramic beads, three cycles of a mechanical homogenization of duration 40 - 60 second, and freeze-thawing process followed by centrifugation, rotary evaporation and lyophilization. The samples were prepared for NMR spectroscopy by dissolving the lyophilized sample in 800 µl of 2H2O. The 600 µl of dissolved sample was added to NMR tube containing the 40 µl of 8 mM reference compound 4, 4-dimethyl-4-silapentane-1-sulfonic acid-d6 (DSS). The final concentration of DSS was 0.5 mM. The data was acquired on a Bruker NMR spectrometer operating at 500 MHz 1H resonance frequency equipped with a cryogenically cooled triple resonance (1H, 13C, 15N) TXI probe5. Identification of metabolite peaks was done through Chenomx and the Human Metabolomic Database (HMDB); finally, the peaks were integrated in Topspin and normalized to the reference compound (DSS). All proton NMR spectra were normalized to the platelet count before analysis. A representative overlaid specta is shown in Figure 1.Results
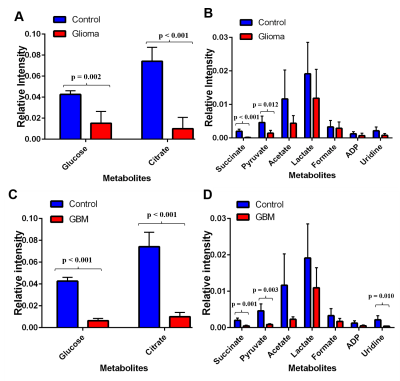
Analysis of 1H-NMR metabolic profiles of low-grade glioma patient platelet samples (n = 10) and glioblastoma patient platelets (n = 10) revealed that glucose, citrate, and succinate are significantly lower in concentration compared to control platelets (n = 4, p < 0.01, Figure 2).Discussion
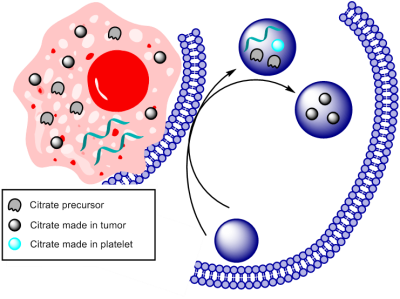
We hypothesized that platelets derived from brain cancer patients exhibit altered metabolism compared to platelets from healthy volunteers. NMR metabolomics has a potential to identify and quantify altered metabolism in platelets collected from brain cancer patients. As depicted in Figure 2 glucose is altered significantly in glioma and GBM patients manifesting reprogrammed metabolic glycolysis pathway. The altered citrate, succinate, and uridine shows not only glycolysis but also the tricarboxylic acid cycle (TCA) and pyrimidine pathways supporting cell proliferation either by providing energy requirements or providing building block moieties. However, it is still needed to be understood how and why platelet’s metabolic reprogramming occurs. We anticipated two ways this mechanism can unfold: I) Exchange of metabolites between tumor and platelets and II) RNA transferred from tumor triggers this metabolic reprogramming Figure 3. Further investigation is necessary to get insight into altered metabolism of platelets derived from brain cancer patients.Conclusion
The obtained results are significant because we detected a metabolic difference related to the progression of brain cancer with an ex vivo NMR based method. The analysis between glioma platelets (n = 10) and glioblastoma platelets (n = 10) suggests that succinate (p = 0.019) can be used as a possible biomarker for brain cancer prognosis. This method is promising because it is analogous to a simple blood test which is both cost-effective and is minimally invasive to the patients. The results need to be compared with other diagnosis techniques as Magnetic Resonance Imaging (MRI), Computerized Axial Tomography (CAT), Positron Emission Tomography (PET), and biopsy (histology) for further validation. Finally, more robust studies should be developed in order to understand the mechanism behind the regulation of key metabolic pathways in the platelets in brain cancer system.Acknowledgements
SP and PB thank the Department of Cancer Systems Imaging and NMR Facility at The University of Texas MD Anderson Cancer Center for imaging resources supported by NIH/NCI CCSG grant P30CA016672. SP acknowledge Computational Cancer Biology Training Program (CCBTP) Fellowship, training grant award RP170593 from the Cancer Prevention & Research Institute of Texas (CPRIT). This research has been funded in part by CPRIT RP 150701, MD Anderson Institutional Research Grants , MD Anderson Institutional Startup, Brain SPORE Developmental Research Award, John S. Dunn Foundation and Koch Foundation.References
1. Jayashree, K.; Manasa, G. C.; Pallavi, P.; Manjunath, G. V., Evaluation of Platelets as Predictive Parameters in Dengue Fever. Indian Journal of Hematology & Blood Transfusion 2011, 27 (3), 127-130.
2. Nolte, I.; Przibylla, H.; Bostel, T.; Groden, C.; Brockmann, M. A., Tumor–platelet interactions: Glioblastoma growth is accompanied by increasing platelet counts. Clinical Neurology and Neurosurgery 2008, 110 (4), 339-342.
3. Nilsson, R. J. A.; Balaj, L.; Hulleman, E.; van Rijn, S.; Pegtel, D. M.; Walraven, M.; Widmark, A.; Gerritsen, W. R.; Verheul, H. M.; Vandertop, W. P.; Noske, D. P.; Skog, J.; Würdinger, T., Blood platelets contain tumor-derived RNA biomarkers. Blood 2011, 118 (13), 3680.
4. Stone, R. L.; Nick, A. M.; McNeish, I. A.; Balkwill, F.; Han, H. D.; Bottsford-Miller, J.; Rupaimoole, R.; Armaiz-Pena, G. N.; Pecot, C. V.; Coward, J.; Deavers, M. T.; Vasquez, H. G.; Urbauer, D.; Landen, C. N.; Hu, W.; Gershenson, H.; Matsuo, K.; Shahzad, M. M. K.; King, E. R.; Tekedereli, I.; Ozpolat, B.; Ahn, E. H.; Bond, V. K.; Wang, R.; Drew, A. F.; Gushiken, F.; Lamkin, D.; Collins, K.; DeGeest, K.; Lutgendorf, S. K.; Chiu, W.; Lopez-Berestein, G.; Afshar-Kharghan, V.; Sood, A. K., Paraneoplastic Thrombocytosis in Ovarian Cancer. New England Journal of Medicine 2012, 366 (7), 610-618.
5. Pudakalakatti, S. M.; Uppangala, S.; D'Souza, F.; Kalthur, G.; Kumar, P.; Adiga, S. K.; Atreya, H. S., NMR studies of preimplantation embryo metabolism in human assisted reproductive techniques: a new biomarker for assessment of embryo implantation potential. NMR in Biomedicine 2013, 26 (1), 20-27.
Figures