0448
Oxidative stress measured by in-vivo, longitudinal 1H MRS and ex-vivo ESR spectroscopy in a rat model of chronic Hepatic Encephalopathy1Center for Biomedical Imaging, EPFL, Lausanne, Switzerland, 2Service of Biomedicine, CHUV, Lausanne, Switzerland, 3Swiss Center for Liver Disease in Children, Department of Pediatrics, HUG, Geneva, Switzerland, 4Laboratory of Physics of Complex Matter, EPFL, Lausanne, Switzerland
Synopsis
Oxidative stress is believed to play a role in the pathogenesis of chronic hepatic encephalopathy (CHE), however no in-vivo studies to assess the course of cerebral oxidative stress in CHE were performed. We investigated the oxidative stress in the hippocampus of a rat model of chronic HE using in-vivo and longitudinal 1H-MRS combined with ex-vivo ESR spectroscopy. Early changes in brain antioxidants (Asc, 4weeks post-BDL), concomitant with Gln and NH4+ changes were detected for the first time in-vivo. These results were confirmed by ESR spectroscopy suggesting that central oxidative stress is an early event in CHE.
PURPOSE
Reactive oxygen species (ROS) are small messenger molecules that are normal components of signal transduction cascades during physiological processes but are also involved in neurotoxicity and neurodegeneration. The redox homeostasis is maintained by the balance between ROS generation and elimination by antioxidants. ROS are critical for hippocampal long-term potentiation, a synaptic plasticity for learning and memory as well as in aging/disease-related impairment1. When in excess ROS lead to cellular dysfunction.
Oxidative stress is believed to play a role in the pathogenesis of chronic hepatic encephalopathy (CHE)2. CHE is accepted to occur in part from glutamine (Gln) accumulation secondary to impaired ammonium (NH4+) clearance by the diseased liver, and Gln accumulation leads to mitochondrial dysfunction. Impaired brain NH4+ detoxification together with Gln increase induces ROS generation in turn associated with astrocyte swelling3,4. To date there are no in-vivo studies to assess the course of cerebral oxidative stress in CHE.
The aim of this study was to investigate the oxidative stress in the hippocampus of a rat model of chronic HE using in-vivo and longitudinal 1H-MRS combined with ex-vivo ESR spectroscopy.
METHODS
In-vivo 1H-MRS was performed on a 9.4T-MR-system (Varian/Magnex-Scientific) using a home-built 14mm-diameter quadrature 1H-surface coil as a transceiver. The SPECIAL sequence5 was used (TE=2.8ms,TR=4sec;160averages). The hippocampus (2x2.8x2mm3) of Wistar male adult rats was scanned before bile-duct ligation (BDL-animal model of CHE) and after every 2weeks up to week8 (n=20). First and second-order shims were adjusted using FASTESTMAP. Concentrations of metabolites were calculated by LCModel using water as reference.
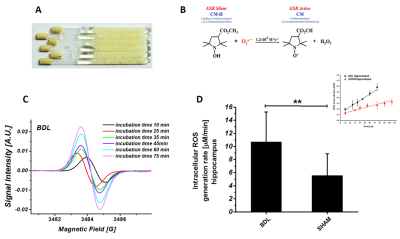
For ESR experiments the hippocampus was extracted at 6weeks post-BDL (n=6) and sham-surgery, weighed, sliced and transferred into whole RPMI1640 medium (Sigma) with 10mM CMH spin trap (Noxygen GmbH), incubated at 37oC. After each incubation time tissue suspension was transferred into quartz capillary tube. Experiments were carried out at RT using an ESP300E spectrometer (Bruker BioSpin GmbH), equipped with a TE102 cavity.
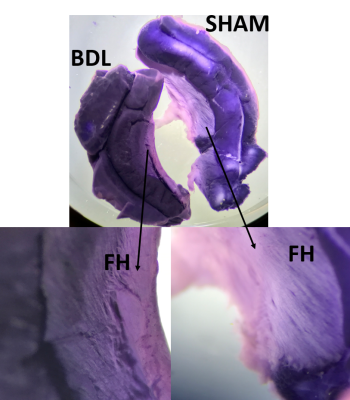
Extracted hippocampus was incubated in the dark with Nitro Blue Tetrazolium (1.6 mg/mL) in HBSS at 37oC for 60 min for histochemical detection of ROS species.
RESULTS AND DISCUSSION
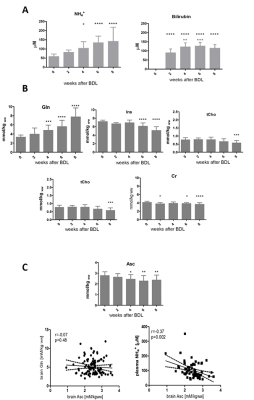
As expected, the presence of chronic liver disease was confirmed by the early increase in plasma NH4+ and bilirubin (2weeks post-BDL). The characteristic neurochemical pattern of CHE was also observed: gradual increase of Gln (significant at week4 post-BDL),significant decrease in main osmolytes (week6 post-BDL) as an osmotic answer to Gln accumulation (Ins-30%, tCho-26%, Tau-8%, Cr -11%) and neurotransmitters decrease (Glu-11%) (Fig.1).
Inositol acts as a multifaceted player in cell signalling and has an important role in maintenance of normal brain function. Neurotransmitters like norepinephrine, dopamine, serotonin, acetylcholine and GABA rely on inositol to relay messages6-9. Alteration in Ins levels influences the calcium pump and in consequence might imbalance the ROS homeostasis10.
Oxidative stress is known to be an important feature of HE. Therefore, we focused on the evolution of two important brain antioxidants, Asc and GSH, using 1H-MRS. Asc showed a statistical significant decrease at week4 compared to week0 (-12%,p<0.05) and reached -15% at week8 (p<0.01). Asc decreased over time correlating mainly with plasma NH4+ increase (r=-0.37,p=0.002) but no correlation was observed with brain Gln (Fig.1), suggesting that the two may be directly linked in the pathogenesis of oxidative stress11 in the central nervous system of rats with CHE. We also observed a trend toward decreasing levels of GSH (-16% at 8weeks post-BDL).
The ESR CMH spin trapping was applied to evaluate ROS levels in hippocampus ex-vivo. CMH is an effective cell permeable and non-toxic spin probe for the quantification of slow released intracellular O2·-. Statistically significant increase of hippocampal ROS levels in BDL (p<0.01, Fig.2) corroborates the 1H-MRS findings of decreasing Asc (Fig.1).
NBT staining reflected increased ROS activity of the fimbria hippocampi (FH) in BDL rats (Fig.3). FH is made of myelinated axons providing the major conduit for subcortical connections, forming fimbria-fornix path12. A significant difference in hippocampus size and weight was observed between shams (120.8±20mg) and BDL animals (71.1±12.7mg)(p<0.01). Therefore, it is tempting to extrapolate that high ROS levels are associated with a loss of cholin-, GABA- and glutamatergic neurons as well as with behavioural deterioration.
CONCLUSIONS
The present study shows the potential to monitor oxidative stress in a rat model of CHE using in-vivo and longitudinal 1H-MRS combined with ex-vivo ESR spectroscopy. We detected, for the first time in-vivo, early changes in brain antioxidants (4weeks post-BDL), concomitant with Gln and NH4+ changes. These results were confirmed by ESR spectroscopy suggesting that central oxidative stress is an early event in CHE.Acknowledgements
Supported by CIBM of the UNIL, UNIGE, HUG, CHUV, EPFL, the Leenaards and Jeantet Foundations and the SNSF project no 310030_173222/1.References
1Lauren T. Knapp and Eric Klann, Journal of Neuroscience Research, 2002; 2Bosoi CR, Rose CF. Metab Brain Dis. 2013 Jun;28(2):175-8; 3Skowronska M. and Albrect J., Neurochemistry International, 2012; 4Lachman V. et al., Archives of Biochemistry and Biophysics, 2013; 5Mlynárik et al, Magn Reson Med 2006; 6Michael J. Berridge, Biochem. Soc. Trans. 2012; 7Boittin, Franc¸ois-Xavier et al., the American Physiological Society,1999; 8Joseph Levine, European Neuropsychopharmacology, 1997; 9Y Ohmori, K Kuriyama, Neurochem. Int., 1989; 10A.K.M. Shamsuddin and Guang-Yu Yang,Inositol & its Phosphates: Basic Science to Practical Applications, 2015; 11Rama Rao et al. 2003; Noremberg 2004; 12The Hippocampus Book, Oxford University Press, 2007; 13 Baehner RL, Boxer LA, Blood, 1976; 14 Armstrong JS et al., Int J Androl. 2002.Figures