0447
Effects of Deuteration on Pyruvate Metabolism in the Isolated Heart1UT Southwestern Medical Center, Dallas, TX, United States, 2University of Texas at Dallas, Richardson, TX, United States, 3Veterans Affairs North Texas Healthcare System, Dallas, TX, United States
Synopsis
Deuteration of metabolites is increasingly popular in hyperpolarization experiments as it prolongs the T1 of the 13C resonances. However, the effect of deuteration on metabolism needs to be investigate to validate the hyperpolarization experiments. Kinetic isotope effects of deuteration of pyruvate were analyzed in isolated rat hearts. No kinetic isotope effect were observed in the pyruvate pool (lactate/alanine) or the TCA cycle (glutamate C5), but a 2H-1H displacement was observed in alanine transaminase and an increase of the contribution of unlabeled sources.
Introduction
Stable isotopes are widely used to investigate metabolism. Since many applications are restricted by the poor sensitivity of 13C and the low concentration of metabolic products, there is strong interest in hyperpolarization methods which temporarily increase 13C polarization by 10,000-fold or more. In recent years, deuteration of metabolites for hyperpolarization experiments has become increasingly popular. Since T1 decay of 13C polarization limits the duration of data acquisition, incorporation of 2H prolongs the T1 of 13C in some molecules, enabling a longer period of observation[1-2]. In particular, perdeuterated glucose and perdeuterated pyruvate have been of interest [3,4].However, 2H significantly influences fluxes in some metabolic pathways. The effect of perdeuteration of glucose on the relative fluxes of glycolysis has been examined. No kinetic isotope effect was observed from glucose to lactate, but a slowing of relative flux was observed into alanine and glutamate. In addition, a wealth of new information was introduced into the system by the formation of deuterated isotopomers, which due to chemical shift isotope effects and extensive quadrupolar couplings allowed additional insight into pyruvate compartmentation and proton-deuteron displacement in alanine transaminase[4]. The effect of deuteration on pyruvate metabolism has not been examined in intact tissues. Here, we investigated the relative rates of metabolism of [2-13C1,3,3,3-2H3]pyruvate through alanine aminotransferase, pyruvate dehydrogenase and lactate dehydrogenase in the isolate rat heart.Methods
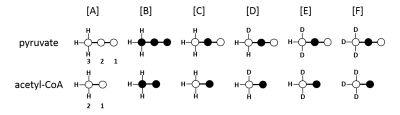
Metabolism of deuterated metabolites were investigated in perfused rat hearts in two studies. A 1:1 mixture of [U-13C3]pyruvate and [2-13C1, 3,3,3-2H3]pyruvate (n=3) or [U-13C3]pyruvate as a control experiment were used (n=3) . Tissue extracts were analyzed by 1H, 2H and 13C NMR spectroscopy. The exensive coupling of the 13C resonances to the quadrupolar deuterium nuclei produces complicated multiplet spectra in glutamate. Therefore, the 13C label was chosen to be in C2 position, to avoid direct coupling and enable a simple analysis of glutamate C5. However, a through-bond chemical shift isotope effect can be expected on the C2 position of lactate and alanine, allowing analysis of the 13C and 2H labeling in the intracellular pyruvate pool.Results & Discussion
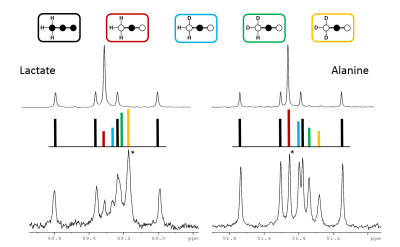
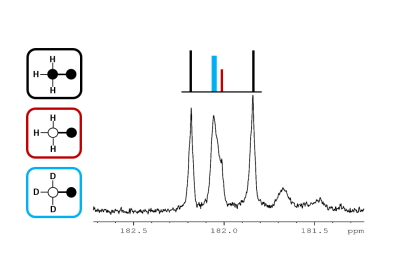
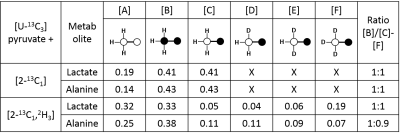
In this experimental design, [U-13C3]pyruvate was used as an internal control and was present in all experiments. The effects of deuteration on metabolism of [2-13C1]pyruvate were examined. The labeling pattern of alanine and lactate were both used to monitor the labeling of intracellular pyruvate. The proton-decoupled 13C NMR spectrum of the control hearts behaved as expected and showed the 1:1 ratio of singlet (from [2-13C1]pyruvate) and quartets (from [U-13C3]pyruvate) in the C2 of both lactate and alanine. A total of seven 13C-labelled isotopomers can be expected in the deuterated experiment (Figure 1). In the 13C NMR spectrum derived from the hearts treated with deuterated pyruvate, the C2 of both lactate and alanine show four singlets of varying intensity and also the quartet derived from [U-13C3]pyruvate (Figure 2). The four singlets represent the four possible species of [2-13C1,3,3,3-2H3]pyruvate, where one, two or three 2H nuclei are replaced by protons and are shifted according to the number of neighboring 2H nuclei. The distribution of species was calculated as previously reported[4]. The resulting distribution between all possible isotopomers for lactate and alanine is shown in Table 1, confirming the deuterium exchange with solvent protons in alanine aminotransferase. The contribution of [2-13C1]pyruvate relative to [U-13C3]pyruvate in the intracellular pyruvate pool was not sensitive to deuteration. However, in the presence of deuterium, the contribution of endogenous glycogen to the intracellular pyruvate pool was increased with a range from 0.19 to 0.32 in lactate and from 0.14 to 0.25 in alanine. The analysis of glutamate was performed at the C5 position (singlet vs doublet) and followed the 1:1 ratio observed in lactate and alanine, meaning PDH flux is not inhibited by the deuteration. Interestingly, the chemical shift isotope effect is often regarded as going upfield. Here, the shift effect has a different sign and is observed downfield (Figure 3), which is likely due to the carbonyl group in glutamate C5.[5]Conclusion
The potential use of perdeuterated pyruvate in hyperpolarization experiments was investigated. Similar to the effects seen when using perdeuterated glucose, the exchange between alanine and pyruvate through alanine aminotransferase introduces exchange of 2H with solvent 1H. However, little effect was observed on relative fluxes into lactate and alanine. The glutamate C5 analysis showed an unusual chemical shift isotope effect, but it follows the same pattern as lactate and alanine. Overall, there is no observable kinetic isotope effect in lactate, alanine and glutmate, confirming the validity for the use of deuterated pyruvate for hyperpolarization experiments.Acknowledgements
This project was supported by NIH P41EB015908 and R37HL034557.References
[1] Mishkovsky M, Anderson BL, Karlsson M, Lerche MH, Sherry AD, Gruetter R, Kovacs Z, Comment A. Measuring glucose cerebral metabolism in the healthy mouse using hyperpolarized 13C magnetic resonance, Scientific Reports, 2017, 7, 11719
[2] Harris T, Degani H, Frydman L. Hyperpolarized 13 C NMR studies of glucose metabolism in living breast cancer cell cultures. NMR Biomed. 2013, 12, 1831-1843 PMID: 24115045.
[3] Vuichoud B, Milani J, Bornet A, Melzi R, Jannin S, Bodenhausen G, Hyperpolarization of Deuterated Metabolites via Remote Cross-Polarization and DIsssolution Dynamic Nuclear Polarization, J.Phys. Chem. B, 2014, 118, 1411-1415.
[4] Funk AM, Anderson BL, Wen X, Hever T, Khemtong C, Kovacs Z, Sherry AD, Malloy CR, The rate of lactate production from glucose in hearts is not altered by per deuteration of glucose, J Magn. Reson., 2017, 284, 86-93. PMID: 28972888
[5] Jameson CJ, Isotope Effects on Chemical Shifts and Coupling Constants, in eMagRes, John Wiley & Sons,Ltd, 2007
Figures