0446
Assessing metabolism and function of normothermically perfused ex vivo livers by multi-nuclear MR imaging and spectroscopy1Oxford Centre for Clinical Magnetic Resonance Research (OCMR), University of Oxford, Oxford, United Kingdom, 2Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom, 3Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, United Kingdom, 4Department of Imaging Methods, Institute of Measurement Science, Slovak Academy of Sciences, Bratislava, Slovakia, 5OrganOx Ltd, Oxford, United Kingdom, 6Institute of Biomedical Engineering, University of Oxford, Oxford, United Kingdom, 7Wolfson Brain Imaging Centre, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom
Synopsis
Liver transplantation is the only cure for end-stage liver disease. Unfortunately, 20% of patients die waiting for a donor. New techniques for preserving transplant livers, such as normothermic machine perfusion (NMP), provide an opportunity to utilise ‘marginal’ (currently discarded) donated livers if their viability can be assessed accurately. We present initial results from a CE-marked NMP system that we adapted for use in an MRI scanner. We demonstrate the power of NMP-MRI to assess structure and metabolism in a freshly donated pig liver, dynamically over a 10-hour period. Our protocol includes 1H imaging, 31P spectroscopy, and hyperpolarised 13C spectroscopy.
Introduction
Liver transplantation is the only definitive treatment for end-stage liver disease, which is otherwise fatal. Waiting lists for liver transplants are increasing rapidly and there is a pressing need to use more “marginal” donated livers that are currently discarded due to the increased risk of graft failure. Recent studies have shown improved short-term post-transplant outcomes following preservation by normothermic machine perfusion (NMP).1 NMP maintains the liver ex situ in an oxygenated, normothermic circuit with nutrient supplementation. However, current technology only provides crude assessment of the viability of marginal livers. To address this we combined a commercial CE-marked NMP device with a 3T MRI scanner. We show that this enables detailed metabolic and structural assessment of livers using 31P-MRS, hyperpolarised 13C-MRS and 1H imaging techniques.Methods
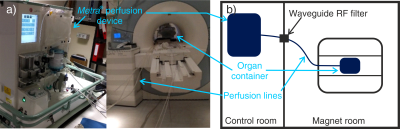
Perfusion system: A CE-marked commercial NMP device (“metra”, OrganOx, UK) was adapted by extending its perfusion circuit through the MRI scanner waveguide, and swapping components in the bore for non-magnetic equivalents (Fig. 1). For validation, a liver was retrieved from a 66kg white landrace pig (as per standard human donor after brain death retrieval) and cold-flushed with University of Wisconsin preservation solution before being stored on ice. The hepatic artery, portal vein, inferior vena cava and bile ducts were all cannulated. Following 4 hours of static cold storage, NMP was initiated as described by Ravikumar et al.2 After two hours of NMP, a 15 minute period of warm ischemia was generated by disconnecting the oxygen supply. Warm ischemia was confirmed by the partial pressure of O2 in the perfusate remaining below 5 kPa.
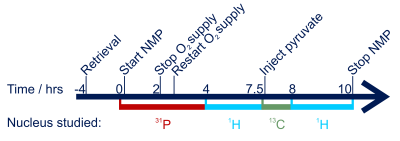
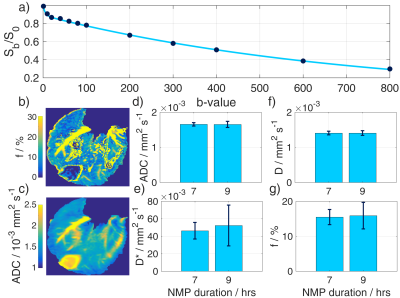
MRI protocol: Scans were performed on a 3T TIM-Trio (Siemens) for ten hours (Fig. 2). 31P-MRS: a 2-channel loop-butterfly coil (Rapid Biomedical, Germany) was used to acquire non-localised FIDs repeatedly for 3.5 hours (TR/TE=3000/0.85 ms, FA=90°, bandwidth=4 kHz, points=2048, averages=200). 13C-MRS: Nonsterile [1-13C]pyruvate was hyperpolarized with EPA radical using a 5T GE Spinlab3 and injected manually into a drug line placed into the hepatic portal vein at the same time as the acquisition of slice-selective FIDs (TR=1 s, FA=10°, bandwidth=5 kHz, points=2048, slice thickness=10 cm). Spectra were acquired using a 10 cm loop coil (PulseTeq, UK). 1H-MRI: A diffusion-weighted spin-echo sequence with echo planar readout with a 32-channel receive array (InVivo Inc, USA), and 12 b-values (0, 10, 20, 40, 60, 80, 100, 200, 300, 400, 600, 800), in 3 directions was acquired.4 TR/TE=2400/65 ms, averages=2, slices=20, voxel size=1.4×1.4×8 mm3. ROIs were then drawn in the right lobe of the liver and signal intensity fitted to quantify perfusion by intravoxel incoherent motion (IVIM):5
$$\frac{S_b}{S_0}=fe^{-bD^*}+(1-f)e^{-bD},$$
where $$$f$$$ is the perfusion fraction, $$$D$$$ is the apparent diffusion coefficient experienced by the nonvascular compartment, and $$$D^*$$$ is the pseudo-diffusion coefficient experienced by the microvascular compartment.
Results
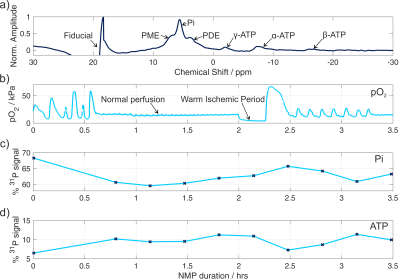
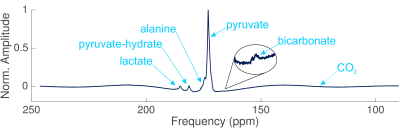
The ATP time course obtained by 31P-MRS (Fig. 3) shows a 2-fold increase in the amount of ATP detected at the start of perfusion, then a gradual decrease following a 15 minute interruption to the oxygen supply. The large oxygen bolus at 2.5 hrs causes another increase in ATP. The inverse trend is seen in the inorganic phosphate (Pi). The ratios of lactate:pyruvate, alanine:pyruvate, and (bicarbonate+CO2):pyruvate following 7.5 hours of NMP were 0.104±0.004, 0.012±0.008, and 0.024±0.005 respectively. The effect of the varying production rates of metabolites can be seen in the sizes of the metabolite peaks in the spectra (Fig. 4) The IVIM results (Fig. 5) show perfusion of all liver tissue and stable perfusion over time with no significant changes between 7 and 9 hours of NMP (P = 0.35).Discussion
This study introduces a new system to examine metabolic processes in ex vivo livers, which are active due to the normothermic machine perfusion. In a proof-of-principle pig liver perfusion, regeneration of ATP was seen at the beginning of the perfusion and during a large oxygen bolus following a period of warm ischemia. The relatively large amount of lactate being produced in the hyperpolarised experiment data, combined with the gradual increase in Pi during stable perfusion, suggests that this liver was partially ischemic. We are further improving the perfusion setup to obtain fully physiological normoxia. Nevertheless, IVIM maps confirm that all regions of the liver were perfused. The next step is to verify and extend our MR-protocols on discarded human livers, for which ethical approval is in place.Conclusions
This study introduces a new normothermic machine perfusion and MRI paradigm for studying liver metabolism ex vivo. This will be useful for optimising future perfusion device design, understanding the mechanisms of preservation and post-perfusion injury in human transplantation and, perhaps, in the long term for assessing the viability of livers for transplantation.Acknowledgements
This work was funded by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society (Grant No. 098436/Z/12/Z) and by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC).References
- Nasralla D, Consortium for Organ Preservation in Europe (COPE) Liver Research Group, Pleog R, et al. Outcomes from a Multinational Randomised Controlled Trial Comparing Normothermic Machine Perfusion with Static Cold Storage in Human Liver Transplantation. Am J Transplant 2017; 17(S3):404
- Ravikumar R, Jassem W, Mergental H, et al. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am J Transplant 2016; 16(6):1779-1787.
- Ardenkjaer-Larsen JH, Leach AM, Clarke N, et al. Dynamic nuclear polarization polarizer for sterile use intent. NMR in Biomedicine 2011; 24(8):927-932
- Leporq B, Saint-Jalmes H, Rabrait C, et al. Optimization of intra-voxel incoherent motion imaging at 3.0 Tesla for fast liver examination. J Magn Reson Imaging 2015; 41(5):1209-1217
- Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986, 161(2):401-407
Figures