0427
Role of hippocampus in genesis of visual hallucinations in Parkinson’s disease: Insights from hippocampal sub-volumetry analysis1Neurology, NIMHANS, Bengaluru, India, 2Symbiosis International University, Pune, India, 3NIMHANS, Bengaluru, India, 4Department of Electronics, Symbiosis Institute of Technology, Symbiosis International University, Pune, India
Synopsis
The neurobiological underpinnings of psychosis, manifested through visual hallucinations (VH) in Parkinson’s disease (PD), are not fully elucidated, however, are linked to memory impairment and are associated with alterations in the hippocampus. To obtain profound understanding of the role of hippocampus in VH, a hippocampal subfield volumetric analysis on PD patients with (PD-P) and without psychosis (PD-NP) and healthy controls (HC) was performed. The results demonstrated a clear pattern with lowest volumes in PD-P, followed by PD-NP and the highest in HCs in several subfields. Moreover, the PD-P volumes in multiple sub-fields highly correlated with memory and attention scores.
Introduction
Psychosis, manifested through formed visual hallucinations (VH) or minor hallucinations, is a common non-motor symptom of Parkinson’s disease (PD). Although numerous risk factors for VH that include older age, female gender, greater disease severity, depression, autonomic dysfunction, sleep disturbances, and cognitive impairment have been identified1, a comprehensive understanding of the neurobiological underpinnings of VH is not fully elucidated.
The hippocampus being a site of unification of spatial and non-spatial contextual information is crucial in encoding and retrieval of event memories2. It has been speculated that inappropriate integration of visual information by the hippocampi could induce hallucinations instead of reflecting reality3. This work therefore aims to obtain a profound understanding of the role of hippocampus in the genesis of VH, by employing a detailed hippocampal subfield analysis on PD patients with (PD-P) and without psychosis (PD-NP), and healthy controls (HC) and tests their associations with cognitive functions.
Methods:
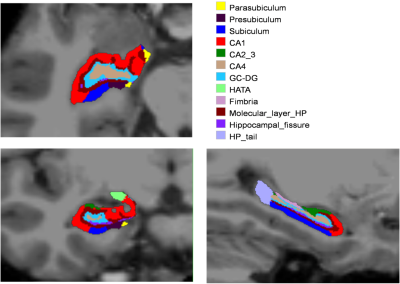
Imaging data on 69 subjects (PD-P: 28, PD-NP: 24 and HC:17, age, gender matched, MMSE > 24) was acquired at using a Philips Achieva® 3T MRI scanner.High-resolution 3D T1 TFE images were acquired with repetition time (TR) = 8.1 ms, echo time (TE) = 3.7 ms, flip angle = 8°, sense factor = 3.5, field of view (FOV) = 256 × 256 × 155 mm, voxel size = 1 × 1 × 1 mm, slice thickness = 1 mm, acquisition matrix = 256 × 256, 165 sagittalslices. An automated subfield parcellation (using FreeSurfer 6.0)4 was performed and volumes of 12 subfields on each side were estimated (figure 1), normalized and analyzed for group differences using a MANCOVA model while co-varying for age and gender. Multiple comparisons correction was carried out using false discovery rates (FDR) at a significance level of 0.1. Further, the volumes were correlated to the neuropsychological tests assessing memory (Rey auditory verbal learning test (RAVLT), visuo-spatial functions (Corsi’s block tapping test), and attention (digit span). The scores were initially adjusted for age and gender by means of linear regressions and the resulting standard residuals were utilized in the correlation. Pearson’s r was computed and the significance of the correlation was maintained at p-value < 0.01.Results:
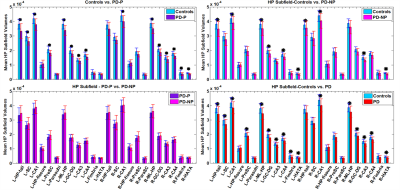
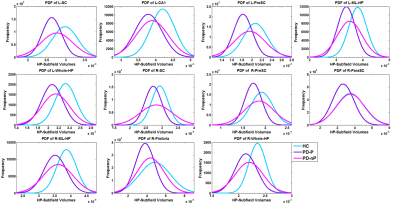
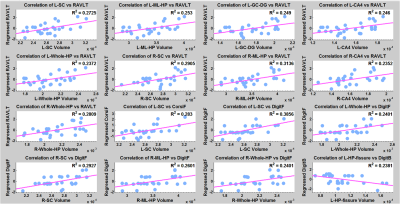
Significant differences in bilateral CA1, hippocampus-amygdala transition area (HATA), left molecular layer, Granule cell-Dentate gyrus (GC-DG), CA4, hippocampal tail, and right CA3 were found in both groups (PD-P and PD-NP) when compared to HC. In addition, left subiculum and presubiculum, and right fimbria had significantly lower volumes in PD-P group (compared to HC). Between PD-P and PD-NP groups, a strong trend of volume deficits were observed in PD-P patients; however,was not significant after correction (figure 2). To attain deeper understanding of the trend between PD-P and PD-NP, we plotted the PDFs of the specific subfield volumes for all 3 groups (figure 3). In majority of the regions (shown in Figure 3)it was observed that the PD-P group had the lowest subfield volumes compared to the other two groups, while the PDF of PD-NP group was located in between the controls and PD-P. Finally, in the correlation analysis, significant positive correlations were observed only in the PD-P group (figure 4): (i) the RAVLT delayed recall score had a significant positive correlation (p-value <0.01) with the volume of bilateral subiculum, molecular layer, GC-ML-DG, CA3, CA4, right presubiculum, and right CA1, (ii) Corsi span (forward) had significant positive (pvalue< 0.01) correlation with volumes of subiculum, presubiculum, and parasubiculum in left side, (iii) digit span (forward) had significant positive (p-value < 0.01) correlation with volume of bilateral subiculum, presubiculum, molecular layer, CA4, fimbria, left GC-ML-DG, (iv) digit span (backward) had negative correlation with left hippocampal fissure volume.Discussion
Our findings indicate higher degeneration of specific hippocampal subfields in patients with PD and psychosis. Although we did not observe statistically significant difference in the volumes of the subfields between the two PD subgroups, there was a clear trend of lower volumes of several subfields in PD-P compared to PD-NP and that of PD-NP compared to the controls. Furthermore, these changes were highly correlated with the cognitive scores implying that structural alterations of hippocampus are perhaps associated with pathogenesis of psychosis in PD and a complex relationship between cognitive impairment and hallucinations may exist. Future studies involving larger dataset may provide better insights into the role of hippocampus in the genesis of VH in PD.Acknowledgements
We would like to thank CDAC BRAF for providing their parallel computing facility as well DST SERB for funding this project (ECR/2016/000808).References
1. Lenka, Abhishek, et al. "Psychosis in Parkinson's disease: From the soft signs to the hard science." Journal of the Neurological Sciences (2017).
2. Behrendt, Ralf-Peter. “Contribution of hippocampal region CA3 to consciousness and schizophrenic hallucinations.” Neuroscience &BiobehavioralReviews 34 (2010) 1121–1136.
3. Lenka, Abhishek, et al. "Structural and functional neuroimaging in patients with Parkinson's disease and visual hallucinations: a critical review." Parkinsonism & related disorders 21.7 (2015): 683-691.
4. Fischl B; FreeSurfer: Neuroimage. 2012 Aug 15;62(2):774-81
Figures