0422
Resting state functional corticostriatal connectivity in Parkinsonian monkeys1Center for Life Science Technologies, RIKEN, Kobe, Japan, 2Okayama University, Okayama, Japan, 3Kyoto University, Kyoto, Japan, 4Department of Neuroscience, Washington University, St. Louis, MO, United States, 5St. Luke's hospital, St. Louis, MO, United States
Synopsis
Studies in animal models of Parkinson’s disease have established that excessive synchronization of neuronal activity in basal ganglia cortical loops is the hallmark of movement disorders in Parkinson’s disease. However, majority of the studies have focused on basal ganglia and motor areas in Parkinson’s disease models and it is still unclear how dopamine denervation influences other neocortical areas, which each exhibit distinct corticostriatal and thalamocortical connectivity profiles. To address this issue, we investigate the effects of dopaminergic neuronal loss on functional connectome using resting-state fMRI in anesthetized MPTP-treated monkeys.
Introduction
Breakdown of information processing in cortico-basal ganglia
circuits is considered to be a hallmark of Parkinson’s disease (PD)1,2.
Electrophysiological studies in PD patients and MPTP-treated primates indicate
excessive synchronization in part of the cortico-basal ganglia circuits, however,
it remains largely unknown how dopamine deficiency modulates converging
corticostriatal network activities. To address this issue, we investigate the effects
of dopaminergic neuronal loss on the functional connectome using resting-state
fMRI in anesthetized MPTP-treated monkeys.
Methods
Our study consists of a total 13 monkeys: controls (N=6), MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine) repeated intramuscular injection (N=6)3 and a single-dose intra-arterial injection (N=1)4. Animals were anesthetized (isoflurane 1.0%) and intubated for spontaneous ventilation. MRI experiments were performed using a 3 T scanner (Siemens, Verio) in combination with a custom-made 16-channel coil. Anatomical images were acquired using T1 MPRAGE (0.5×0.5×0.5 mm mm, TI 800 ms, TR 2500 ms). Resting-state scans were acquired with gradient-echo EPI (TR 760 ms, 1.5×1.5×1.8 mm, TE 30 ms, 150 minutes per animal). Data analysis used the Non-Human primate Human Connectome Project (NHPHCP) pipelines5. These processes transformed the data into a standard set of grayordinates (164k and 10k for structural and fMRI, respectively) in the dense Connectivity Informatics Technology Initiative (CIFTI) format. FMRI data was denoised using FIX (aggressive regression of 24 movement parameters and manual classification of noise components with non-aggressive regression of these) for structural artefacts6. Dual regression of spatial and temporal ICA network templates7, which were generated using a separate data set, was performed to obtain estimates of resting-state networks (RSN) in each subject. Statistically significant differences between the groups were determined using FSL’s PALM permutation t-test. Wishart roll-off denoised dense timeseries8 were parcellated to generate 201 node timeseries9. The functional connections (edges) between these nodes were estimated using correlation and Tikhonov-regularized (rho 0.1) partial correlations10. Dopamine function was assessed using PET (microPET, Siemens, Focus 220) with dopamine transporter ligand, 11C-PE21 (N-(3-iodoprop-2E-enyl)-2β-carbomethoxy-3β-(4-methyl-phenyl)nortropane) for the animal exposed to a single-dose MPTP injection (N=1). Partial volume effects were corrected for using a region-based-voxel method11 and binding potential was calculated using SRTM2 with cerebellum as the reference tissue12.Results
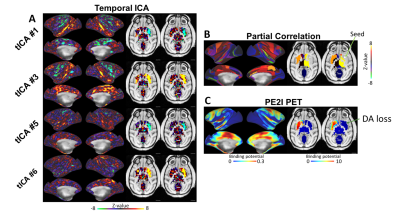
Corticostriatal functional connectivity in MPTP group was markedly altered (Fig. 1 and 2). Spatially orthogonal RSNs (spatial ICA) showed most prominent changes in premotor-putamen and prefrontal-caudate networks (Fig. 1A and B, respectively). Temporally orthogonal RSNs (temporal ICA) exhibited more diverse functional changes throughout the cortex, including higher visual - motor auditory (Fig.2A), default mode (Fig. 2B), cortico medial structure - sensorimotor (Fig. 2C), dorsal sensorimotor (Fig.2 D) networks. To specifically examine corticostriatal connectivity we next examined parcellated connectivity using 'full' and 'partial' correlations (Fig. 3). Statistically significant (p < 0.01, Bonferroni corrected) connectivity increases was observed between the putamen and F1, 7m and area 31, whereas decreases were observed from visual areas (V1, V2 and V3). Mean (absolute) cortico-putamen connectivity estimated with 'full' correlation increased 2-fold (Fig. 4A, p<0.001) and partial correlation increased by 20% (Fig. 4B, p<0.001). A hemiparkinsonian monkey showed marked lateral changes in functional connectivity (Fig. 5A,B) ipsilateral to dopamine denervation confirmed with PE2I PET (Fig. 5C).
Discussion & conclusions
We have shown that dopaminergic neuronal loss amplifies corticostriatal functional connectivity. These results are in line with our findings using dopaminergic modulation of the functional connectome13. Importantly, the hemiparkinsonian results (Fig. 5) indicate that these connectome modulations are likely to be neural as there is no reason to believe that changes in physiological noise or effects of anesthesia would alter the connectome of only half of the brain. We propose that elevated corticostriatal and intra- and interhemispheric striatal correlations are a consequence of increased convergent information flow in PD14 (Fig. 4C). These findings corroborate the hypothesis that dopamine supports segregation of functional corticostriatal subcircuits1, and extend this view to a temporally-distinct low-frequency band in which cortical inputs converge in the basal ganglia of patients with PD.
Acknowledgements
We thank Prof. Van Essen DC in Washington University School of Medicine for providing the Non-Human Primate Connectome Pipeline. This research is partially supported by the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from Japan Agency for Medical Research and development, AMED, by MEXT KAKENHI Grant (16H03300,16H03306, 16H01626, 15K12779).References
1Bergman et al (1998) Trends in Neuroscience 21, 32-38. 2Haber (2016) Dialogues in Clinical Neuroscience 18. 3Saiki et al (2010) J. Neuroscience method 190, 198-204. 4Hayashi et al (2013) Journal of Clinical Investigations 123, 272-284. 5Glasser et al (2013) Neuroimage 80, 105–124. 6Griffanti et al (2014) Neuroimage 95, 232-247. 7Smith et al (2012) PNAS 109, 3131-3136. 8Glasser et al (2016) Nature 53, 171-178. 9Markov et al (2011) Cerebral Cortex 21, 1254-1272. 10Smith et al (2016) Nature Neuroscience 18, 1565-1567. 11Greve et al (2016) Neuroimage 132, 334-343. 12Wu et al (2002) JCBFM 22, 1440-1452. 13Nishigori et al (2017) Organization for Human Brain Mapping, Vancouver, #2216. 14Berkson (1946) Biometrics Bulleting 2, 47-53.
Figures