0419
Findings from SNAP-MRA and PCASL in Patients with Parkinson’s Disease “OFF” and “ON” Levodopa1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Tsinghua University Yuquan Hospital, Beijing, China, 3Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China
Synopsis
This study aimed to evaluate the effect of levodopa on cerebral arteries and gray matter cerebral blood flow (CBF) in patients with Parkinson’s disease (PD). We scanned 33 PD patients with “OFF” and “ON” levodopa using Simultaneous Non-Contrast Angiography and intraPlaque Hemorrhage (SNAP) MRA and Pseudo-Continuous Arterial Spin Labeling (PCASL), and used multiple statistical methods to analyze the data. Results from the statistical analysis show that after taking levodopa, patient’s treatment outcomes are highly correlated with his/her gray matter CBF. The results also suggest that levodopa can dilate cerebral arteries and improve CBF, but only in patients with mild symptoms.
Purpose
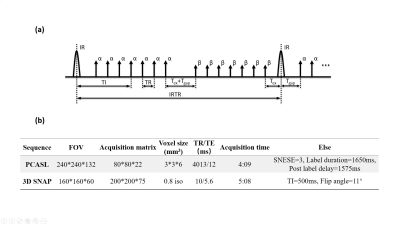
The diagnosis and treatment of Parkinson’s diseases (PD) have gained greater attention for clinical researchers. Previous studies have reported cerebral hemodynamic changes and perfusion disturbances in PD patients1-4. This study focused on the effect of levodopa5 (the most commonly used drug in PD treatment) on cerebral perfusion in PD patients. We used Pseudo-Continuous Arterial Spin Labeling (PCASL)6 to quantify the cerebral blood flow (CBF) changes before and after taking levodopa. On the other hand, Simultaneous Non-Contrast Angiography and intraPlaque Hemorrhage (SNAP)7 is an artery imaging technique (Fig. 1a) which can provide a full 3D luminal magnetic resonance angiography (MRA) and an intrinsically registered 3D intraplaque hemorrhage (IPH) image in a single scan7. SNAP-MRA can provide intracranial vascular images with effective background suppression offered by the phase-sensitive reconstruction technique8. Its potential in intracranial vascular stenosis detection has been shown9. In this study, we used SNAP-MRA to observe the cerebral arteries changes before and after taking levodopa, and investigated the relationship with CBF changes and clinical manifestations.Methods
Participants and data acquisition 33 patients diagnosed with PD (age, 57.8±9.9 years; male/female, 16/17) were recruited from Tsinghua University Yuquan Hospital, Beijing, China and provided with informed consent. Patients were told to stop taking drugs 12 hours before the first scan (“OFF” levodopa). After the first scan patients took levodopa (~300 mg), and they were scanned use the same protocol for the second time 50 minutes later (“ON” levodopa). Before each scan, the Unified Parkinson’s Disease Rating Scale 3.0 (UPDRS-III) for each patient was scored by an experienced specialist. All scans were performed on a Philips 3.0T Achieva TX MR scanner (Philips Healthcare, Best, The Netherlands) using an 32-channel SENSE head coil. The detailed scan parameters are listed in Fig. 1b. Finally, we collected “OFF” and “ON” UPDRS scores and an “OFF” image dataset for each patient. 25 patients had “ON” image datasets.
Data post-processing The SNAP images were zero-padded in all directions to achieve 0.4 mm isotropic resolution, then the phase-sensitive reconstruction and maximum intensity projection (MIP) were implemented to generate background suppressed SNAP-MRA. The SNAP-MRA images were assessed by two experienced specialists and scored with two two-point scales: for branch vessel length, 0=short and 1=long; for arterial morphology, 0=thin and 1=thick. The whole-brain CBF maps were calculated from perfusion weighted images using the PCASL model10. Gray matter (GM) masks were segmented from T1-weighted anatomical images using SPM811. The CBF maps were registered to the GM masks and the mean values of the GM CBF were calculated. Statistical analysis was conducted in GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA) to explore the correlations among “OFF” and “ON” levodopa SNAP-MRA findings, GM CBF and the UPDRS scores.
Results and Discussion
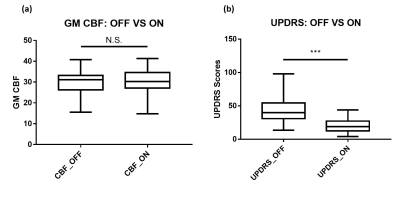
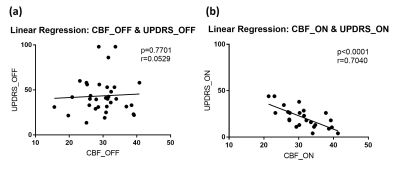
The paired t-test shows significant difference (p<0.0001) between the UPDRS scores from “OFF” and “ON” levodopa, which demonstrates the efficacy of levodopa (Fig. 2b). However, GM CBF from “OFF” and “ON” levodopa have no obvious difference (p=0.7613) (Fig. 2a). The linear regression analysis shows no correlation (p=0.7701, r=0.0529) between UPDRS_OFF and CBF_OFF, but strong correlation (p<0.0001, r=0.7040) between UPDRS_ON and CBF_ON (Fig. 3). These suggest that the patient’s short-term treatment outcomes are highly correlated with his/her “ON” CBF values.
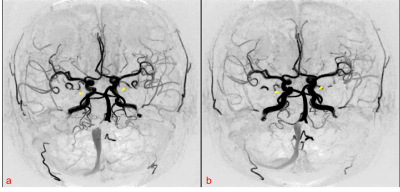
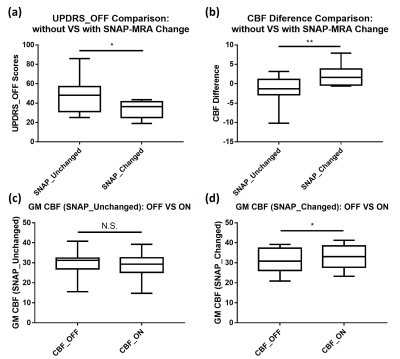
For SNAP-MRA from “OFF” and “ON” levodopa, the Chi-square test shows that the branch vessel length has no marked change (χ2=0.005, P=0.945) while the arterial morphology changes significantly (χ2=4.463, P=0.035). 12 of 25 “ON” SNAP-MRA have visual morphological changes (labeled as SNAP_changed) like dilated arteries and enhanced image intensity (Fig. 4). Further analysis which combined SNAP-MRA and PCASL findings shows that SNAP_changed patients had remarkably lower UPDRS_OFF, which means they had mild symptoms, and their CBF values were enhanced markedly after taking levodopa (Fig. 5).
Many previous works5, 12, 13 have reported that levodopa is effective in the early stages of PD, but its drug resistance and side effects become serious as the disease progresses during the long term therapy. Based on the results above we can draw a preliminary conclusion: levodopa can dilate cerebral arteries and increase CBF in PD patients with mild symptoms, but this feature is lost as the disease progresses and its efficacy decreases during the long term PD therapy.
Conclusion
In this study, we tried to evaluate the effect of levodopa on cerebral arteries and GM CBF in PD patients. The results show that patient’s short-term treatment outcomes are highly correlated with his/her “ON” GM CBF. Levodopa can dilate cerebral arteries and improve GM CBF, but only in PD patients with mild symptoms.Acknowledgements
No acknowledgement found.References
[1] Jahanshahi M, Jenkins I H, Brown R G, et al. Self-initiated versus externally triggered movements: I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects[J]. Brain, 1995, 118(4): 913-933.
[2] Firbank M J, Colloby S J, Burn D J, et al. Regional cerebral blood flow in Parkinson's disease with and without dementia[J]. Neuroimage, 2003, 20(2): 1309-1319.
[3] Fernández-Seara M A, Mengual E, Vidorreta M, et al. Cortical hypoperfusion in Parkinson's disease assessed using arterial spin labeled perfusion MRI[J]. Neuroimage, 2012, 59(3): 2743-2750.
[4] Teune L K, Renken R J, de Jong B M, et al. Parkinson's disease-related perfusion and glucose metabolic brain patterns identified with PCASL-MRI and FDG-PET imaging[J]. NeuroImage: Clinical, 2014, 5: 240-244. [5] Parkinson Study Group. Levodopa and the progression of Parkinson's disease[J]. N Engl J Med, 2004, 2004(351): 2498-2508.
[6] Garcia D M, De Bazelaire C, Alsop D. Pseudo-continuous flow driven adiabatic inversion for arterial spin labeling[C]//Proc Int Soc Magn Reson Med. 2005, 13: 37.
[7] Wang J, Börnert P, Zhao H, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation[J]. Magnetic resonance in medicine, 2013, 69(2): 337-345.
[8] Kellman P, Arai A E, McVeigh E R, et al. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement[J]. Magnetic resonance in medicine, 2002, 47(2): 372-383.
[9] Wang J, Guan M, Yamada K, et al. In Vivo Validation of Simultaneous Non-Contrast Angiography and intraPlaque Hemorrhage (SNAP) Magnetic Resonance Angiography: An Intracranial Artery Study[J]. PloS one, 2016, 11(2): e0149130.
[10] Alsop D C, Detre J A, Golay X, et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia[J]. Magnetic resonance in medicine, 2015, 73(1): 102-116.
[11] Ashburner J, Barnes G, Chen C, et al. SPM8 manual[J]. Functional Imaging Laboratory, Institute of Neurology, 2008, 41.
[12] Marsden C D, Parkes J D. Success and problems of long-term levodopa therapy in Parkinson's disease[J]. The Lancet, 1977, 309(8007): 345-349.
[13] Katzenschlager R, Lees A J. Treatment of Parkinson's disease: levodopa as the first choice[J]. Journal of neurology, 2002, 249.
Figures