0405
Europium(2+/3+) dual mode MRI contrast agents: combining paraCEST and T1w contrast in one oxygen-sensitive agent.1UT Southwestern Medical Center, Dallas, TX, United States, 2University of Texas at Dallas, Dallas, TX, United States
Synopsis
The ability to determine hypoxia in tumors in vivo could provide useful diagnostic contrast information. MRI contrast agents could provide this information. Eu2+ is isoelectronic with Gd3+, and produces T1w contrast on a similar level, in addition, it is not oxidatively stable. In an aerobic atmosphere, it oxidizes rapidly to Eu3+, which does not produce T1 contrast, but can belong to a conceptually different class of contrast agents: paraCEST (chemical exchange saturation transfer) agents. A Eu2+/3+ agent was tested in different tissues in-vivo to show the correlation between the rate of oxidation and the surrounding oxidative environment of the tissue.
Introduction
Oxygen is an essential part of tissue health and cell survival. It plays a vital role in the treatment of many medical conditions, including tumors, peripheral vascular disease and stroke. Tumors are typically hyper-metabolic and are often deprived of oxygen. In addition, hypoxia is known to affect the radiation sensitivity of tumors and promote development of metastases. The ability to determine hypoxia in tumors in vivo could provide useful diagnostic information. Gd3+ complexes are routinely used as contrast agents in magnetic resonance imaging (MRI). These agents generate image contrast by shortening the longitudinal (R1) relaxation rate of the bulk water protons, but are usually not responsive to physiological changes. Eu2+ is isoelectronic with Gd3+, and produces T1w contrast on a similar level to Gd3+. Unfortunately, in an aerobic environment, Eu2+ is rapidly oxidized to Eu3+, which does not have favorable paramagnetic properties for a T1 proton relaxation.[1] However, Eu3+ can produce image contrast by a conceptually different class of contrast agents, called paraCEST (chemical exchange saturation transfer) agents[2]. These agents alter image contrast by transferring selectively saturated spins to bulk water pool via chemical exchange.[1] The rate of oxidation of Eu2+ to Eu3+ can be manipulated somewhat by its coordination environment.[2] By designing an agent that slowly oxidizes in tissues but once oxidized becomes an efficient paraCEST agent, it is possible to create a dual-mode contrast agent that is sensitive to its oxygen environment. Previously, we have shown the in-vivo and in-vitro capabilities of Eu2+/3+-DOTA(gly)4 (Figure 1).[3-4] We have shown that the agent is stable throughout oxidation and showed both T1w contrast and paraCEST in muscle tissue for about 20 minutes. Here, we injected the agent into tumor tissue that is known to have lower oxygen content to investigate the potential of Eu2+/3+-DOTA(gly)4 as an oxidatively sensitive contrast agent to study hypoxia in tumors.Methods
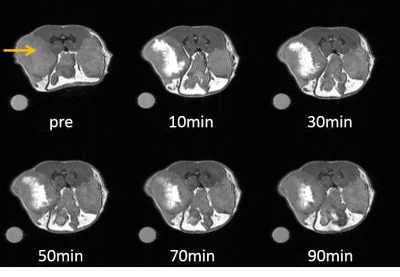
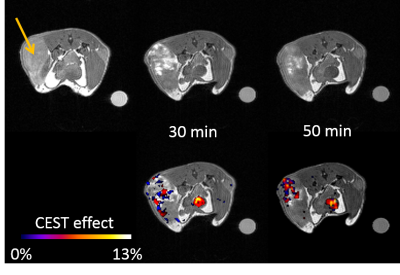
The paraCEST agent 1,4,7,10 tetraazacyclododecane-1,4,7,10-tetraacetic acid tetra(glycine amide) was synthesized as reported previously[5] and was evaluated in-vivo using a Varian 4.7 T small animal imaging scanner. The animals were anesthetized with isofluorane (1-2% medical oxygen) and the agent was injected intratumoral (10mM, 100μL). The T1w images were obtained using a ge3D sequence (TR=3.6ms, TE=1.8ms), while the CEST images were obtained using a modified GEMS sequence (sat power=15 μT, sat time=3 s, sat frq= 42 (on resonance), -42 (off resonance). NOD-SCID mice were used to implant A549 cells subcutaneously and to grow tumors with a diameter of 5mm to 1cm (n=5).Results & Discussion
We have previously shown that oxidation of Eu2+-DOTA(gly)4 (T1 contrast agent) to Eu3+-DOTA(gly)4 (paraCEST agent) proceeds without the loss of metal ion from the ligand and the T1 contrast last for about 20 minutes in muscle tissue. The partial oxygen pressure, pO2, characterizes the oxygen content of tissues and the rate of oxidation of Eu2+ should be directly related to the pO2 of the tissue. Compared to muscle tissue (pO2 = 30 mmHg), tumor tissue has a lowered pO2 (<10 mmHg for the core and 10-20 mmHg for outer parts)[6]. Therefore, the rate of oxidation should be drastically lowered in tumor tissue. Indeed, at the core of the tumor, the T1 effect lasted almost 90 minutes, while at the edges and under the skin it oxidized much faster (Figure 2), but overall showing the expected reduction in the rate of oxidation compared to the muscle injections. Furthermore, a CEST effect was observed at the injection site that prevailed at the injected site for a prolonged time (Figure 3). In addition, CEST was visible in the bladder after 30 minutes. To further confirm the tumor heterogeneity, the tumor was extracted and was further investigated using histological staining, which confirmed a higher degree of hypoxia at the core of the tumor.Conclusion
Depending on the oxidative environment, the rate of oxidation of the Eu2+/3+ agent is drastically altered, from 20 minutes of T1 contrast to almost 90 minutes with a change of only 30 mmHg to <10 mmHg partial oxygen pressure, pO2. By injection the Eu2+/3+ agent into different tissue types, it was confirmed that the rate of oxidation can me modified by the surrounding oxidative environment. This could allow determination of the degree of hypoxia in tumors through injection of the Eu2+/3+ agent.Acknowledgements
This project was supported by NIH R01-CA115531 and P41EB015908.References
(1) Viswanathan S, Kovacs Z, Green K N, Ratnakar S J, Sherry A D, Chem. Rev. 2010, 110 (5), 2960–3018.
(2) Burai L, Tóth É, Moreau G, Sour A, Scopelliti R, Merbach A E, Chem. – Eur. J. 2003, 9 (6), 1394–1404
(3) Funk A M, Clavijo-Jordan V, Sherry A D, Ratnakar S J, Kovacs Z, Angew. Chem. 2016, 128 (16), 5108–5111.
(4) Burnett E B, Adebesin B, Funk A M, Kovacs Z, Sherry A D, Ekanger L A, Allen M J, Ratnakar S J, Eur. J. Inorg. Chem. 2017, in Press.
(5) K. N. Green, S. Viswanathan, F. A. Rojas-Quijano, Z. Kovacs, A. D. Sherry, Inorg. Chem., 2011, 50, 1648-1655;
(6) Carreau, A.; Hafny-Rahbi, B. E.; Matejuk, A.; Grillon, C.; Kieda, C. J. Cell. Mol. Med. 2011, 15 (6), 1239–1253.
Figures