0404
Multimodality cellular and molecular imaging of the impact of a primary tumor on metastatic growth in a syngeneic mouse model of breast cancer brain metastasis1Medical Biophysics, Western University, London, ON, Canada, 2Western University, London, ON, Canada
Synopsis
The mechanisms that influence metastatic growth rates are poorly understood. One mechanism of interest known as concomitant tumor resistance (CTR) can be defined as the inhibition of metastatic growth by existing tumor mass. Conversely, the presence of a primary tumor has also been shown to increase metastatic outgrowth, termed concomitant tumor enhancement (CTE). The goal of this research was to use conventional and cellular MRI , and bioluminescence imaging to study the impact of a primary tumor on the development of breast cancer brain metastases in a syngeneic mouse model.
Introduction
Metastasis is responsible for the majority of cancer-related deaths and mechanisms that control metastasis are poorly understood. One mechanism of interest called concomitant tumor resistance (CTR) refers to the ability of the primary tumor to restrict the growth of distant metastases1,2. Removal of a primary tumor can be followed by abrupt acceleration of residual metastatic disease, and has been observed in both animal models of breast cancer3 and patients4. Conversely, a primary tumor can likewise increase metastatic outgrowth, a phenomenon coined concomitant tumor enhancement (CTE). CTE has been reported in the clinic, with most cases being related to suspected regressions of hepatic or pulmonary metastases following nephrectomy for renal cell carcinoma5-8. While imaging has been used to describe CTR/CTE effects in patients9, the majority of studies evaluating CTR/CTE in preclinical models have relied on histological evaluation of tumor burden10,11. The application of cellular and molecular imaging tools capable of visualizing metastatic progression in vivo will yield a better understanding of the mechanism(s) by which CTR/CTE effects occur and under what conditions. In turn, this may lead to new therapeutic approaches to halt metastatic outgrowth. Here we apply iron-oxide-based cellular MRI and bioluminescence imaging (BLI) to study the effects of a primary tumor and its size on metastatic growth of breast cancer cells in a novel syngeneic mouse model.Methods
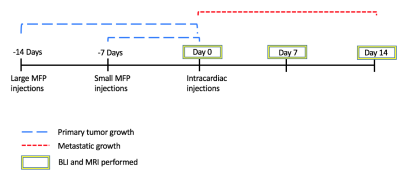
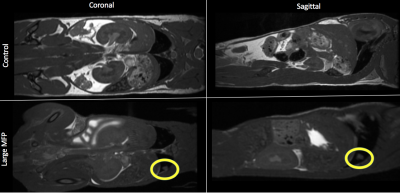
BALB/c mice (n=24) received an injection of vehicle (Control) or 3x105 parental 4T1 cells in the mammary fat pad (MFP) either 7 days (small MFP) or 14 days (large MFP) prior to intracardiac injection of 2x104 luciferase-expressing, iron-labeled brain-seeking 4T1BR5 cells (Figure 1). Cellular MRI and BLI were performed over the next 2 weeks to measure brain and whole-body cancer cell viability (BLI), whole-brain single cell arrest (iron-induced MR signal voids), and the number and volume of metastases at endpoint (MRI). Whole body MRI was performed on large MFP and control mice on days 9 and 14. BLI was performed on an optical imaging scanner and MRI was performed on a 3T scanner using customized gradient and solenoidal RF coils using the iron-sensitive bSSFP sequence.Results
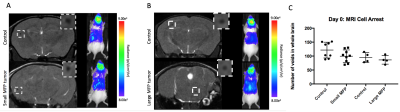
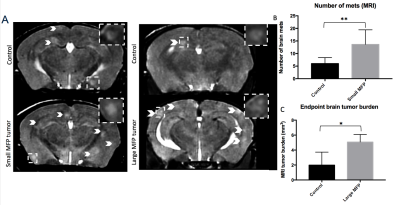
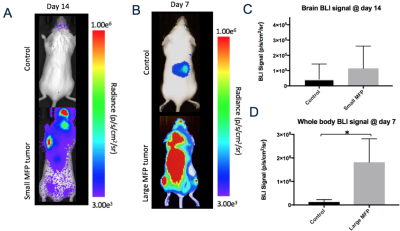
Iron labeled cells were visualized in brain MR images as discrete signal voids on day 0 (arrested cells) which was not significantly different between Control and MFP mice (Figure 2). Brain BLI signal at day 0 was also not significantly different (Figure 2). At day 14, both small and large MFP mouse groups had significantly more brain metastases (p<0.05) and brain tumor burden (p<0.05) than Control mice (Figure 3). Whole-body and brain BLI signal at endpoint were not significantly different between small MFP mice and Control mice, but were significantly higher for the large MFP group compared to Control mice (Figure 4). Lung metastases were detectable at day 14 with whole body MRI in mice with a large MFP primary tumor but not Control mice (Figure 5).Discussion
The mechanisms of CTR and CTE remain unclear. These findings are in contrast to our previous study in immune compromised mice where we found the presence of a human MDA-MB-231 breast tumor significantly inhibited the growth of MDA-MB-231BR brain metastases. Other groups have suggested the immune system can play a crucial role in CTR/CTE effects12. Furthermore, previous studies have shown that the size of the primary tumor can play a key role in whether a CTE or CTR effect is observed13. For the present study, we injected our metastatic cell line at an early time point (day 7) when the primary tumor was relatively small and a late time point (day 14) when the primary tumor was large. We found the presence of a primary tumor enhances the growth of brain and body metastases and secondly, this effect could be amplified by increasing the size of the primary tumor at the time of secondary injection. Using in vivo BLI/MRI we could determine this was not related to differences in initial arrest or clearance of viable cells in the brain, which suggests that the presence of a primary tumor can increase the proliferative growth of brain metastases in this syngeneic mouse model.Conclusion
For the first time, we have applied cellular and molecular imaging tools to evaluate the effect of a primary breast tumor on the growth of brain metastases in an immune competent model. Our work highlights new insights into the effects a primary tumor can have on breast cancer metastasis. Understanding the molecular mechanisms behind stimulation (CTE) versus inhibition (CTR) of metastatic growth could lead to new targets for therapy that could prevent recurrence and improve patient outcome.Acknowledgements
No acknowledgement found.References
1. Chiarella P, Bruzzo J, Meiss RP, Ruggiero RA. Concomitant tumor resistance: Cancer Lett. 28: 324(2): 133-41, 2012.
2. Prehn RT. The inhibition of tumor growth by tumor mass. Cancer Res. 51: 2-4, 1991.
3. McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 2008; 133:994-1005.
4. Demicheli R, Miceli R, Moliterni A, et al. . Breast cancer recurrence dynamics following adjuvant CMF is consistent with tumour dormancy and mastectomy-driven acceleration of the metastatic process, Ann Oncol , 2005, vol. 16 (pg. 1449-1457)
5. Vizel, M.W. Oster, J.H. Austin, Spontaneous regression of a pulmonary metastasis after nephrectomy for renal cell carcinoma, J. Surg. Oncol. 12 (1979) 175–180.
6. J. Lokick, Spontaneous regression of metastatic renal cancer. Case report and literature review, Am. J. Clin. Oncol. 20 (1997) 416–418.
7. M. Wyczolkowski, W. Klima, W. Bieda, K. Walask, Spontaneous regression of hepatic metastases after nephrectomy and mastectomy of renal cell carcinoma, Urol. Int. 66 (2001) 119–120.
8. K. Lekanidi, P.A. Vlachou, B. Morgan, S. Vasanthan, Spontaneous regression of metastatic renal cell carcinoma: case report, J. Med. Case Reports 1 (2007) 89.
9. El Saghir, N. S., Elhajj, I. I., Geara, F. B., & Hourani, M. H. (2005). Trauma-associated growth of suspected dormant micrometastasis. BMC cancer, 5(1), 94.
10. Kirstein, J. M., Hague, M. N., McGowan, P. M., Tuck, A. B., & Chambers, A. F. (2016). Primary melanoma tumor inhibits metastasis through alterations in systemic hemostasis. Journal of Molecular Medicine, 94(8), 899-910.
11. Franco, M., Bustuoabad, O. D., Di Gianni, P. D., Goldman, A., Pasqualini, C. D., & Ruggiero, R. A. (1996). A serum-mediated mechanism for concomitant resistance shared by immunogenic and non-immunogenic murine tumours. British journal of cancer, 74(2), 178.
12. Janik P, Bertram JS, Szaniawska B. Modulation of lung tumor colony formation by a subcutaneously growing tumor. J Natl Cancer Inst 1981; 66:1155-8.
13. Bruzzo J, Chiarella P, Meiss RP, Ruggiero RA. Biphasic effect of a primary tumour on the growth of secondary tumour implants. J Cancer Res Clin Oncol 2010; 136:1605-15.
Figures