0401
Noninvasive imaging of transgene expression using CEST-MRI1Molecular Imaging Research Center (MIRCen), Commissariat à l'Energie Atomique, Fontenay-aux-Roses, France, 2US27, Inserm, Fontenay-aux-Roses, France
Synopsis
Gene therapy often uses viral vectors to insert a therapeutic gene into cells. It is necessary to develop molecular imaging techniques to safely monitor gene expression. We designed a strategy to image expression of a transgene using a genetically engineered CEST reporter. An adeno associated vector encoding for 150 L-arginine residues fused to a fluorophore was produced and injected in the mouse brain. Specific signals were observed in both CEST and fluorescence modalities, demonstrating the possibility to use CEST-based reporter genes to monitor transgene distribution and expression. Such noninvasive imaging modality could be valuable for further developments of gene therapy.
INTRODUCTION
Gene therapy has experienced tremendous developments during the past decade. This therapeutic approach requires the insertion of therapeutic genes into cells in order to replace defective genes or to complete their expression. The transfer of nucleic acids is often performed using viral vectors derived from adeno associated virus (AAV). Noninvasive imaging of a reporter gene would be an extremely useful tool for monitoring gene delivery and long term expression in vivo. Recently, Chemical Exchange Saturation Transfer (CEST) has been proposed to image diluted molecules with a good spatial resolution1. In particular, nonmetallic polypeptides are good biocompatible candidates and they exhibit huge amplification factors2,3. Contrarily to iron-based reporter genes, CEST contrast is switchable so CEST agents do not modify MRI signal when saturation pulse is off4. A first prototype of CEST-based reporter gene has already been produced but gene transfection was performed in vitro in 9-L rat glioma cells and then, cells were implanted into the brain3. Here, we designed a genetically engineered CEST reporter expressing 150 arginine residues directly in the living brain and we evaluated its expression using CEST-MRI in vivo.METHODS
AAV vector
AAV vector (AAV2/8) expressing poly-L-arginine (PLR) 150R-td-Tomato (150 L-arginine residues fused to the fluorophore td-Tomato) under the control of the PGK (phosphoglycerate kinase) promoter was produced to drive transgene expression into neurons. Mice (n = 4) were anesthetized with a mixture of ketamine (150 mg/kg) and xylazine (10 mg/kg) and received unilateral stereotaxic injections (left or right striatum, n = 2 for each side) of 5x1011 viral genomes in a total volume of 2 µl per injection site.
MRI acquisitions
Data were acquired on a horizontal 11.7T Bruker magnet with a cryoprobe. 1 month after AAV injection, mice were anesthetized using 1.5 % isoflurane in a 1:1 gas mixture of air/O2 and positioned in a dedicated stereotaxic frame. CEST images were acquired using TSE sequence preceded by a continuous wave saturation pulse of 1 s with an intensity B1 of 2.5 µT applied at frequencies ranging from -5 to 5 ppm with a step of 0.25 ppm. B0 inhomogeneity was corrected using WASSR method5. CEST images were calculated using asymmetric Magnetization Transfer Ratio (MTRasym) at ±1.75 ppm.
Histology
Mice were euthanized and perfused with 100 ml of cold 4% paraformaldehyde (PFA) solution in phosphate buffer. Brains were cryoprotected in a 30% sucrose solution in PB for 24 h. Coronal brain sections (35 µm) were cut on a freezing microtome and observed under a fluorescent microscope.
RESULTS
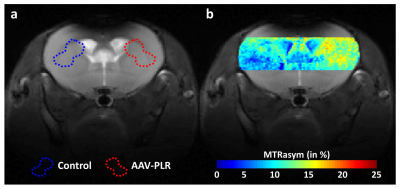
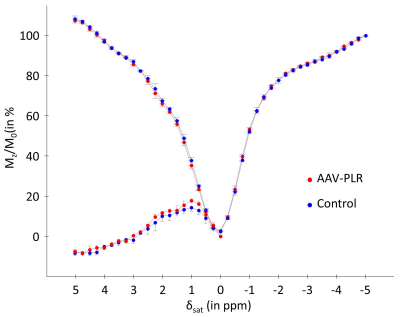
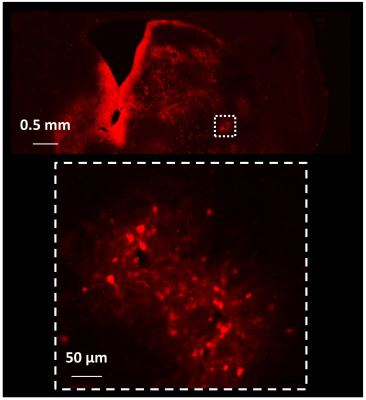
One month after AAV-PLR injection, CEST images acquired at 1.75 ppm showed significant increase of CEST signal in the injected striatum. An example is shown on Fig.1.b. ROIs were manually drawn in each mouse brain to calculate corresponding Z-spectrum and MTRasym in Control and AAV-PLR injected hemisphere (Fig.2, blue and red curves respectively, mean ± SD, n = 4). Z-spectrum and MTRasym acquired in the AAV-PLR injected hemisphere exhibited specific and significant peaks compared to Control spectra, especially in regions comprised between 0.75 to 1 ppm and 1.75 to 2.25 ppm. This can be attributed to poly-L-arginine expressed by the transgene. Fluorescence images (Fig.3) confirmed transfection of neuronal cells by our AAV vector encoding for 150 L-arginine residues fused to the fluorophore.DISCUSSION
All CEST images acquired in the 4 mice showed higher CEST contrast in the hemisphere injected with the AAV. As already described in a pioneer study about polypeptides CEST agents2, arginine-based peptides show strong and specific signals at ~0.8 ppm and ~1.8 ppm corresponding to hydroxyl (-OH) and guanidyl (-NH2) protons. It is consistent with peaks observed in these regions of our Z-spectrum (Fig.2, red curves). We did not observe specific peak at ~3.7 ppm (-NH), probably because MTRasym was dominated by endogenous APT and NOE contrasts6. Interestingly, areas of higher CEST contrast seemed to match with intense fluorescence signals. However, one can also observe some fluorescence signals which were not visible on the CEST image. This can be explained by partial volume effects due to the CEST image slice thickness (1 mm compared to 35 µm for histological section).CONCLUSION
We observed for the first time gene expression following AAV injection into the brain using a CEST reporter gene in vivo. Obviously, further works will be necessary to define the specific contribution of L-arginine to the CEST signal. This proof-of-principle study opens the door to designing new CEST proteins for use as MRI reporter genes in vivo to follow expression of therapeutic transgenes.Acknowledgements
No acknowledgement found.References
1. Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). Journal of Magnetic Resonance. 2000;143(1):79-87.
2. McMahon MT, Gilad AA, DeLiso MA, Berman SM, Bulte JW, van Zijl PC. New "multicolor" polypeptide diamagnetic chemical exchange saturation transfer (DIACEST) contrast agents for MRI. Magn Reson Med. 2008;60(4):803-812.
3. Gilad AA, McMahon MT, Walczak P, et al. Artificial reporter gene providing MRI contrast based on proton exchange. Nature biotechnology. 2007;25(2):217-219.
4. Gilad AA, Ziv K, McMahon MT, van Zijl PC, Neeman M, Bulte JW. MRI reporter genes. J Nucl Med. 2008;49(12):1905-1908.
5. Kim M, Gillen J, Landman B, Zhou J, van Zijl P. Water Saturation Shift Referencing (WASSR) for Chemical Exchange Saturation Transfer (CEST) Experiments. Magnetic Resonance in Medicine. 2009;61(6):1441-1450.
6. Jin T, Wang P, Zong X, Kim SG. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magn Reson Med. 2013;69(3):760-770.
Figures