0400
Layer specific neural interactions in the thalamo-cortical and cortico-cortical networks: An optogenetic manganese-enhanced MRI studyKarim El Hallaoui1,2, Eddie C. Wong1,2, Xunda Wang1,2, Alex T. L. Leong1,2, Russell W. Chan1,2, Celia M. Dong1,2, and Ed X. Wu1,2
1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China
Synopsis
The thalamo-cortical projections terminating in the primary somatosensory cortex are anatomically organized into layers. However, the functional layer-specificity of the axonal pathways which comprise the thalamo-cortical circuit remains to be studied. Combining optogenetic stimulation and manganese-enhanced MRI, the present study selectively induces neural activity in the ventral posteromedial nucleus to induce the deposit of manganese in its projections in-vivo. By identifying the cortical regions with increased contrast, using images acquired with the MDEFT MRI experiment, we infer the layer-specific connections from the thalamus to the primary somatosensory cortex.
Purpose
Anatomical maps of long-range axonal projections from the thalamus to the specific layers of cortical brain regions have been studied extensively1-4. These layers are classified by their respective neuronal characteristics into six distinct categories, layers I through VI (Figure 1A). However, it has been demonstrated that relative axonal densities and synaptic strengths may not adequately characterize the functional connectome of the thalamo-cortical projections4-6. We propose a combination of optogenetic stimulation and manganese-enhanced MRI (MEMRI) as a means to map long-range neural tracts with increased layer-specificity in-vivo. Manganese is a paramagnetic ion which reduces T1 relaxation times; its presence enhances the contrast of images acquired during T1-weighted MRI experiments7,8. Furthermore, manganese has a similar ionic radius to calcium and can traverse ionic channels during neural activity. Therefore, it is a useful method for high resolution and specificity tracing of axonal projections9-12. We suggest that using optogenetics, a tool which can be used to generate spatiotemporally specific neuronal activity13, manganese uptake can be selectively driven to a region of interest14. In this study, we employ in-vivo targeted optogenetic stimulation at the ventral posteromedial nucleus (VPM) to identify the layer-specific radiations to the ipsilateral and contralateral primary somatosensory cortex (S1) through the thalamo-cortical and cortico-cortical networks respectively.Methods
Animal preparation and optogenetic stimulation: AAV5-CaMKIIα::ChR2(H134R)-mCherry was expressed in VPM thalamocortical excitatory neurons in normal Sprague-Dawley rats (n=6; optogenetic). Four weeks after injection, an optic fiber was implanted chronically (diameter=450μm) at the injection site (Figure 1A; center). Additionally, the optic fiber implantation surgery was performed on naive age-matched Sprague-Dawley rats (n=4; sham control). Following one week of rest, the animals were anesthetized and administered MnCl2 by intraperitoneal injection (100mM, 40mg/kg) and allowed to recover for four hours. Subsequently, the animals received optogenetic stimulation (473nm, 40mW/mm2) for four hours using a paired-pulse paradigm (Figure 1B).MEMRI protocol and data analysis: Ten hours after the MnCl2 injection, modified driven equilibrium Fourier transform (MDEFT) images (TR/TE=4000/4.2ms, flip angle=10°, FOV=3.2x3.2 cm2, matrix=256x256 and sixteen contiguous 1mm slices) were acquired with a 7T Bruker scanner. The layers of the S1 are defined based on the rat brain atlas (Figure 1A; right). The MDEFT images are normalized for comparison.
Results
The intraperitoneal manganese injection causes general uptake across the entire brain. Figure 2 shows six MDEFT scan slices from a representative animal which received optogenetic stimulation and a control animal ten hours after injection. The animal which expressed ChR2 at the VPM showed increased uptake in both the ipsilateral and contralateral S1 when compared to that of the control group. The observed uptake is selectively driven by the optogenetic stimulation which induces the propagation of manganese ions to the projections of the VPM.Figure 3 demonstrates the long-range pathways traversed by manganese during targeted optogenetic stimulation of the VPM. This stimulation induces increased concentrations of manganese to be present in the ipsilateral S1 due to activity in the ionic channels of the thalamo-cortical network. Furthermore, it is observed that manganese uptake extends to the contralateral S1. The layer specificity of the manganese uptake in the contralateral S1 for the ten animals (n=6 optogenetic vs. n=4 sham control) involved in this work is presented in Figure 4. The layer-specific increase in contrast can be seen to be centered along layer IV and V for the optogenetic animals.
Discussion and Conclusion
Our study shows the potential of using optogenetic stimulation to selectively drive manganese enhancement and produce MRI images with heightened contrast. We used this technique to study the layer-specific projections onto the S1 through the thalamo-cortical and cortico-cortical networks. A four hour, paired-pulse stimulation, resulted in an increased accumulation of manganese in layers IV and V of the contralateral S1 for animals which received optogenetic stimulation in comparison to the sham control group. It has been reported that stimulation of the ipsilateral S1 induces activity in the contralateral S1 concentrated about the granular cells (layer IV) through the inter-hemispheric cortico-cortical radiations across the corpus callosum10,16,17. It should be noted that this study was conducted in-vivo and the animals moved freely between the time of injection and the MRI experiment. Thus, control animals are expected to have manganese uptake, including in the sensory cortices, albeit at a lesser extent than those which received targeted VPM optogenetic stimulation. In conclusion, combining optogenetics and MEMRI can be a valuable method for mapping the functional connectivity of long-range projections between brain regions with higher spatial resolution in-vivo.Acknowledgements
This work was supported by the Hong Kong Research Grant Council (Grants C7048-16G and HKU17103015 to E.X.W.).References
- Mountcastle, V. B. (1957). "Modality and topographic properties of single neurons of cat's somatic sensory cortex." J Neurophysiol 20(4): 408-434.
- Somogyi, P., et al. (1998). "Salient features of synaptic organisation in the cerebral cortex." Brain Res Brain Res Rev 26(2-3): 113-135.
- Douglas, R. J. and K. A. Martin (2004). "Neuronal circuits of the neocortex." Annu Rev Neurosci 27: 419-451.
- Feldmeyer, D. (2012). "Excitatory neuronal connectivity in the barrel cortex." Front Neuroanat 6: 24.
- Constantinople, C. M. and R. M. Bruno (2013). "Deep cortical layers are activated directly by thalamus." Science 340(6140): 1591-1594.
- Yu, X., et al. (2005). "In vivo auditory brain mapping in mice with Mn-enhanced MRI." Nat Neurosci 8(7): 961-968.
- Cory, D. A., et al. (1987). "Ingested manganese chloride as a contrast agent for magnetic resonance imaging." Magn Reson Imaging 5(1): 65-70.
- de Sousa, P. L., et al. (2007). "Manganese-enhanced magnetic resonance imaging (MEMRI) of rat brain after systemic administration of MnCl2: changes in T1 relaxation times during postnatal development." J Magn Reson Imaging 25(1): 32-38.
- Silva, A. C. and N. A. Bock (2008). "Manganese-enhanced MRI: an exceptional tool in translational neuroimaging." Schizophr Bull 34(4): 595-604.
- Tucciarone, J., et al. (2009). "Layer specific tracing of corticocortical and thalamocortical connectivity in the rodent using manganese enhanced MRI." Neuroimage 44(3): 923-931.
- Massaad, C. A. and R. G. Pautler (2011). "Manganese-enhanced magnetic resonance imaging (MEMRI)." Methods Mol Biol 711: 145-174.
- Chan, K. C., et al. (2014). "In vivo visuotopic brain mapping with manganese-enhanced MRI and resting-state functional connectivity MRI." Neuroimage 90: 235-245.
- Leong, A. T., et al. (2016). "Long-range projections coordinate distributed brain-wide neural activity with a specific spatiotemporal profile." Proc Natl Acad Sci USA 113(51): E8306-E8315.
- Yizhar, O., et al. (2011). "Optogenetics in neural systems." Neuron 71(1): 9-34.
- Paxinos, G. and C. Watson (1998). The rat brain in stereotaxic coordinates, Academic Press, San Diego.
- Baek, K., et al. (2016). "Layer-specific interhemispheric functional connectivity in the somatosensory cortex of rats: resting state electrophysiology and fMRI studies." Brain Struct Funct 221(5): 2801-2815.
- Keller, C. J., et al. (2014). "Mapping human brain networks with cortico-cortical evoked potentials." Philos Trans R Soc Lond B Biol Sci 369(1653): 20130528.
Figures
Figure
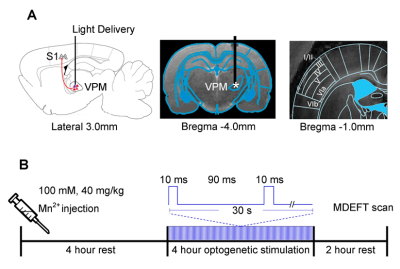
1. Illustration of the optogenetic stimulation
setup in the ventral posteromedial nucleus (VPM). (A) Illustration of the stimulation site in ChR2-expressing VPM
thalamocortical excitatory neurons (left). T2-weighted anatomical MRI image
showing the location of the implanted optical fiber (asterisk: stimulation site;
center). Laminar organization of the primary somatosensory cortex (S1) is based
on the rat brain atlas15 (right). (B) Experiment timeline for the 10 hours preceding the MDEFT scan.
The paired-pulse optogenetic stimulation is presented in continuum for a period
of four hours.
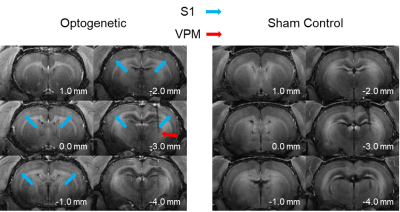
Figure 2. Representative MDEFT images of an optogenetic and sham control animal ten hours after the intraperitoneal injection of manganese. Manganese uptake can be observed across the entire brain. The optogenetic stimulation is induced at the VPM (red arrow) which increases the manganese concentration at the ipsilateral and contralateral somatosensory cortex (S1; blue arrows).
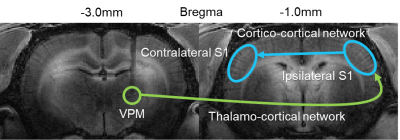
Figure 3. Visualization of the long-range somatosensory thalamo-cortical (green
arrow) and interhemispheric cortico-cortical (blue arrow) network. Neural
activity in the VPM induces manganese uptake to accumulate in the ipsilateral
S1 which then traverses the corpus callosum to reach the contralateral S1.
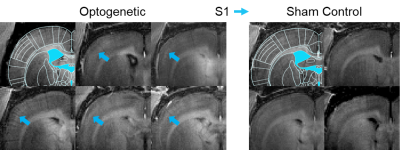
Figure 4. Axial
cross-section MDEFT image (Bregma -1mm) focused on the contralateral somatosensory
cortex for the individual animals involved in this study. Increased manganese uptake is observed (blue
arrows) in the somatosensory cortex of animals which received optogenetic stimulation
at the VPM (left). Specifically, layer IV and layer V of S1 shows increased
contrast with respect to the surrounding region; thus implying higher
concentrations of manganese uptake in these layers.