0399
Active Targeting of human neutrophil granulocytes by non-invasive 19F MRI1Heinrich-Heine University, Düsseldorf, Germany, 2Albert-Ludwigs-University, Freiburg, Germany
Synopsis
The purpose of the present study was to target human neutrophils by PFCs to enable their visualization by 19F MRI. We coupled a neutrophil-binding peptide (NG2) to PFCs and showed the specific binding and internalization of NG2-PFCs by 19F MRI, microscopy and FACS. Interestingly, NG2-PFCs show an increased labelling of neutrophils from MI patients and these cells also showed an increased migration into an artificial circulation system which contained an IL-8 doped matrigel placed in a flow chamber. In conclusion, NG2-PFCs are suited to label neutrophil granulocytes after MI and enable their non-invasive visualization by 1H/19F MRI.
Introduction
Multiple studies in clinically relevant disease models have shown that combined 1H/19F MRI is suitable to visualize inflammatory hot spots with high sensitivity and specificity1-3. As 19F label, perfluorocarbon nanoemulsions (PFCs) are used which are intravenously injected and are taken up predominantly by monocytes and macrophages. However, this technique does not provide full information about the status of the inflammatory process, since neutrophil granulocytes are the first cells that infiltrate inflammatory lesions. This cell population critically impacts on the following healing and resolution process, e.g. in non-healing or chronic inflammatory lesions permanently elevated levels of neutrophils are found. Specific monitoring of neutrophil granulocytes by 19F MRI therefore would provide a more detailed picture of the ongoing immune response and may enable a non-invasive, background-free assessment of the healing status and/or the identification of chronic inflammatory lesions. Therefore, the present study aimed to specifically target and track human neutrophil granulocytes by the use of actively targeted PFCs.Methods
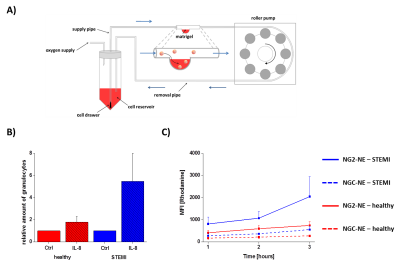
To target human neutrophil granulocytes, utilized neutrophil-binding (NG2) and control (NGC) peptides4 modified with an N-terminal carboxyfluorescein and a C-terminal GGG-spacer followed by a cysteine residue for coupling reactions. Peptides were linked to PFCs (20% PFCE) which contained maleimide-PEG2000-DSPE (0.05 mol%) and also rhodamine-DHPE (0.025 mol%). The resulting NG2/NGC-PFCs were purified and the specific binding/uptake of NG2-PFCs was investigated by flow cytometry, confocal microscopy and 19F MRI. To compare the infiltration kinetics of neutrophils from healthy volunteers and patients with myocardial infarction (MI; 12-24 post MI) into foci of inflammation, we developed an artificial circulation system equipped with a flow chamber that contained a matrigel-based inflammatory hot spot (Fig. 3A). For MRI experiments, the isolated immune cells were incubated with NG2/NGC-PFCs at 37 °C, washed, and separated by percoll density gradient centrifugation. Thereafter, samples were analyzed by 1H/19F MRI at 9.4T using a 25-mm 1H/19F birdcage resonator and standard 1H/19F RARE sequences (19F RARE: TR 2500 ms, FOV = 2.56 ´ 2.56 cm2, matrix: 32 ´ 32, 5 mm slice thickness, 512 averages, 21 min). To investigate the infiltration of neutrophils into foci of inflammation, matrigel was mixed with/without IL-8 (0.1 ng/µl). Thereafter, the isolated immune cells were transferred into the circulation system (flow rate: 120 µl/min), followed by injection of NG2/NGC-PFCs. After circulation for one to three hours, cells were extracted from the matrigel and analyzed by FACS.Results
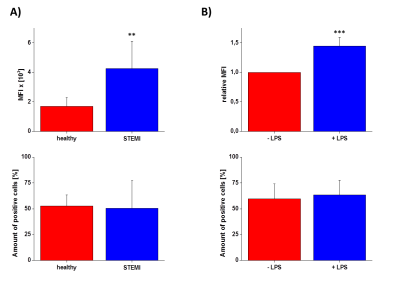
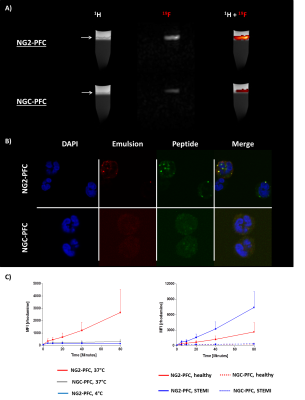
First, we studied the binding specificity of the NG2 vs. the NGC peptide and found that NG2 exclusively bound to a sub-fraction of human neutrophils (52.7±10.7%) but not to monocytes or lymphocytes. Interestingly, neutrophils stimulated with LPS or derived from patients after MI show an increased binding of the NG2 peptide compared to controls (Fig. 1). Furthermore, NG2 turned out to be highly specific for human neutrophils, since we did not observe any binding to neutrophils from rat, mouse or pig. In the next step, we evaluated the binding and internalization of the NG2/NGC-PFCs by 19F MRI. After incubation of neutrophils with NG2/NGC-PFCs, the cells were separated via density gradient centrifugation and subjected to 1H/19F MRI measurements which revealed a significant increase of the 19F signal (3.8 ± 1.4 fold) for NG2-targeted PFCs as compared to the NGC-PFC control. These results were confirmed by flow cytometry and confocal microscopy which revealed that NG2-PFCs (but not NGC-PFCs) were specifically bound and internalized (Fig. 2). We also found that the process of NG2-PFC targeting is energy dependent since incubation at 4 °C completely blocked the incorporation of NG2-PFCs. To mimic the physiological human situation, we investigate the invasion of neutrophils into foci of inflammation, using an in-house developed artificial circulation system which also contained an IL-8 doped matrigel placed in a flow chamber (Fig. 3B). As expected, IL-8 significantly increased in the amount of infiltrated neutrophils from healthy patients. However, neutrophils from patients with MI showed a much stronger infiltration into the focus of inflammation. To track the migrating neutrophils, we injected NG2/NGC-PFCs into the flow system and found that granulocytes from MI patients which infiltrated into IL-8 doped matrigel also displayed a strongly increased labelling of NG2-PFCs (Fig. 3C).Conclusion
Here, we could demonstrate that NG2-PFCs specifically label human neutrophil granulocytes. Trapping of the contrast-cargo within the target cell is ensured by internalization of NG2-PFCs. Importantly, neutrophils from patients with MI show an increased uptake of NG2-PFCs as well as in enhanced migration into a matrigel/IL8-based inflammatory hot spot. Taken together, NG2-PFCs seem to be suited for tracking the invasion of neutrophil granulocytes after myocardial infarction and their non-invasive visualization by 1H/19F MRI.Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG), subproject B02 of the Sonderforschungsbereich 1116.References
1. Hitchens, T. K. et al. 19F MRI detection of acute allograft rejection with in vivo perfluorocarbon labeling of immune cells. Magnetic resonance in medicine 65, 1144–1153 (2011).
2. Ebner, B. et al. Early assessment of pulmonary inflammation by 19F MRI in vivo. Circulation. Cardiovascular imaging 3, 202–210 (2010).
3. Flögel, U. et al. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation 118, 140–148 (2008).
4. Luca Mazzucchelli, James B. Burritt, Algirdas J. Jesaitis, Asma Nusrat, Tony W. Liang, Andrew T. Gewirtz, Frederick J. Schnell and Charles A. Parkos. Cell-Specific Peptide Binding by Human Neutrophils. blood, 1738–1748 (1999).
Figures