0394
True laminar resolution fMRI of the human visual cortex at 7T1Department of Cognitive Neuroscience, Maastricht University, Maastricht, Netherlands, 2Maastricht Brain Imaging Centre (MBIC), Maastricht, Netherlands
Synopsis
Current laminar BOLD fMRI studies at ultra-high field are typically carried out at sub-millimetre spatial resolution (~0.7mm isotropic), which, however, results in each voxel covering more than one cortical layer. Thus, layer-resolved activation profile can only be obtained if such data is analysed with post-processing tools at super-resolution. In this study, we demonstrate a novel functional mapping approach by acquiring fMRI data at true laminar resolution (100µm) in humans at 7T, compare it to the conventional high-resolution GE-EPI and analyse the depth-dependent BOLD signal change to visual stimulation.
Introduction
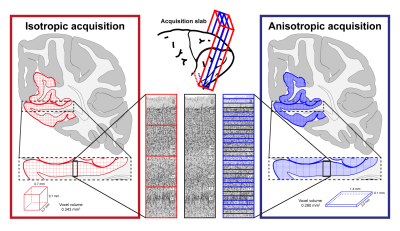
Cortical laminar-resolved fMRI links invasive animal electrophysiology with cognitive neuroscience in humans1. Increasing interest in laminar fMRI has resulted in the development and optimisation of novel acquisition2,3 and analysis approaches4,5 at sub-millimetre spatial resolutions at 7T. Due to the fact that cortical layers occupy a much finer spatial scale than the typical voxel sizes6, the “large” sub-millimetre voxels at 7T along the laminar direction are inadequate to directly resolve laminar signal (Fig. 1, red). Existing approaches address this issue by super-sampling the laminar signal with post-processing tools. However, the layer-specific signal crammed together during acquisition cannot be fully recovered during post-processing. Hence, we propose to forego the isotropicity of voxel resolution and acquire fMRI data at a very high spatial resolution (100µm) along the laminar direction (i.e. at a “true” laminar resolution (Fig. 1, blue)), omitting the need to determine laminar profiles via interpolation and upsampling. Here, we show, for the first time, that it is possible to image and measure meaningful BOLD profiles at a true laminar resolution in vivo in humans.Methods
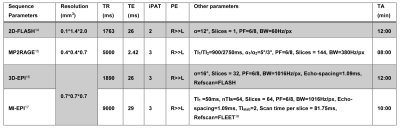
Data was acquired on a Siemens 7T research scanner using a 16ch phased-array visual cortex coil7. Four subjects participated in this study after written informed consent. Full sequence parameters can be found in Fig. 2. Stimulus paradigm: Full contrast flickering checkerboard was presented using PsychoPy8 for 20s followed by 40s of isoluminant grey background. Each functional run had ten blocks. Three anisotropic (i.e. 100µm in the laminar direction; called anisotropic FLASH) and two isotropic (0.7 mm; called isotropic GE-EPI) runs were acquired. Acquisition specification: Placement of the acquisition volume was based on previously acquired anatomical data on a subject-by-subject basis, the region-of-interest (ROI) was defined as a flat portion of the calcarine sulcus (therefore, V1) that was oriented such that the phase-encoding is perpendicular to the laminar direction (Fig. 1, 3). Data analyses: All functional data were motion corrected using ANTs9. Isotropic data: The MI-EPI T1 map was registered to the functional EPI data and further statistical analysis was done without distortion correction4. Laminar analyses was carried out using the CBS-Tools10,11. Anisotropic data: The MP2RAGE T1 was aligned to 2D-FLASH using ITK-SNAP v3.612, registered using ANTs and further laminar analyses was carried out in MATLAB.
Results
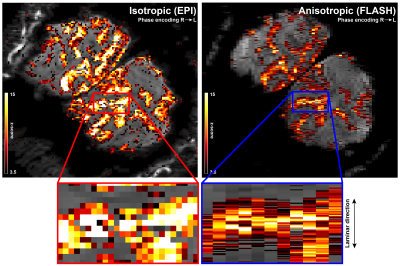
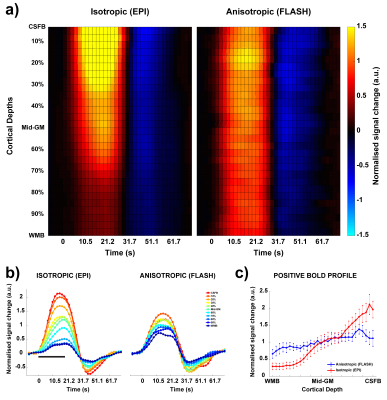
The anisotropic FLASH acquisition method yields robust activation even with a single run (Fig. 3, right) and has enough SNR for reliable laminar analysis, with similar activation ROI as the isotropic acquisition (Fig. 3, left). Fig. 4a shows the event-related average laminar distribution of the BOLD signal sampled by interpolation from the isotropic GE-EPI data and from the true laminar resolution anisotropic FLASH data. The profiles were obtained over the same functional ROI. Fig. 4b shows event-related laminar BOLD signal time-courses (a subset of the laminar signal distribution from Fig. 4a). Even though the general shapes of the time courses appear similar, there are some striking differences between anisotropic and isotropic data (e.g. time to peak, amplitude, post-stimulus undershoot). The laminar positive BOLD response profiles (Fig. 4c) from the isotropic EPI and the anisotropic FLASH profile both show a steady increase in signal change towards the CSF boundary but exhibit different slopes.Discussion
In this study, we demonstrate, for the first time, the feasibility for acquiring BOLD fMRI data at a true laminar resolution in vivo in humans at 7T. We compare this with the current workhorse of laminar fMRI: isotropic GE-EPI. We observe the characteristic GE BOLD signal increase to the pial surface13 in both acquisition approaches. However, the isotropic EPI profile has a steeper slope compared to the true laminar resolution FLASH approach (Fig. 4a and 4c). This can be explained by: a) the fact that the laminar signal is super-sampled from the “low”-resolution isotropic EPI voxels by interpolation, resulting in smoothing of the underlying laminar data, and partial volume effects of the strong GE BOLD signal of the pial vessels and weak GE BOLD signal near the white matter; b) the susceptibility effect of the pial venous signal drops off quadratically with cortical depth and influences the intra-voxel dephasing leading to a BOLD signal, which is not caused by the local vasculature1. However, this “leakage” is relatively less in the anisotropic FLASH approach due to the high spatial resolution in the laminar direction and, hence, more homogeneous field disturbance by the pial vessels. In summary, the anisotropic FLASH approach shows great promise as to achieve hitherto highest laminar spatial specificity in vivo at 7T (or columnar specificity for an orthogonal acquisition scheme).Acknowledgements
The research was supported by the Netherlands Organization for Scientific Research (NWO) VIDI grants 452-11-002 (KU) and 016-178-052 (BAP).References
- Uludağ, K. & Blinder, P. Linking brain vascular physiology to hemodynamic response in ultra-high field MRI. NeuroImage (2017). doi:https://doi.org/10.1016/j.neuroimage.2017.02.063
- Huber, L., Uludağ, K. & Möller, H. E. Non-BOLD contrast for laminar fMRI in humans: CBF, CBV, and CMRO2. NeuroImage (2017). doi:https://doi.org/10.1016/j.neuroimage.2017.07.041
- Yu, X., Qian, C., Chen, D. Y., Dodd, S. J. & Koretsky, A. P. Deciphering laminar-specific neural inputs with line-scanning fMRI. Nat Methods 11, 55–58 (2014).
- Kashyap, S., Ivanov, D., Havlicek, M., Poser, B. A. & Uludag, K. Impact of acquisition and analysis strategies on cortical depth-dependent fMRI. NeuroImage (2017). doi:10.1016/j.neuroimage.2017.05.022
- Polimeni, J. R., Renvall, V., Zaretskaya, N. & Fischl, B. Analysis strategies for high-resolution UHF-fMRI data. NeuroImage (2017). doi:10.1016/j.neuroimage.2017.04.053
- Yen, C. C.-C., Papoti, D. & Silva, A. C. Investigating the spatiotemporal characteristics of the deoxyhemoglobin-related and deoxyhemoglobin-unrelated functional hemodynamic response across cortical layers in awake marmosets. NeuroImage (2017). doi:10.1016/j.neuroimage.2017.03.005
- Sengupta, S. et al. A Specialized Multi-Transmit Head Coil for High Resolution fMRI of the Human Visual Cortex at 7T. PLoS One 11, e0165418 (2016).
- Peirce, J. W. PsychoPy--Psychophysics software in Python. J Neurosci Methods 162, 8–13 (2007).
- Avants, B. B., Tustison, N. J., Wu, J., Cook, P. A. & Gee, J. C. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics 9, 381–400 (2011).
- Bazin, P.-L. et al. A computational framework for ultra-high resolution cortical segmentation at 7Tesla. NeuroImage 93, 201–209 (2014).
- Waehnert, M. D. et al. Anatomically motivated modeling of cortical laminae. NeuroImage 93 Pt 2, 210–220 (2014).
- Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 31, 1116–1128 (2006).
- Koopmans, P. J., Barth, M. & Norris, D. G. Layer-specific BOLD activation in human V1. Hum Brain Mapp 31, 1297–1304 (2010).
- Haase, A., Frahm, J., Matthaei, D., Hanicke, W. & Merboldt, K.-D. FLASH imaging. Rapid NMR imaging using low flip-angle pulses. J. Magn. Reson. 67, 258–266 (1986).
- Marques, J. P. et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage 49, 1271–1281 (2010).
- Poser, B. A., Koopmans, P. J., Witzel, T., Wald, L. L. & Barth, M. Three dimensional echo-planar imaging at 7 Tesla. NeuroImage 51, 261–266 (2010).
- Renvall, V., Witzel, T., Wald, L. L. & Polimeni, J. R. Automatic cortical surface reconstruction of high-resolution T1 echo planar imaging data. NeuroImage 134, 338–354 (2016).
- Polimeni, J. R. et al. Reducing sensitivity losses due to respiration and motion in accelerated echo planar imaging by reordering the autocalibration data acquisition. Magn. Reson. Med. 75, 665–679 (2016).
Figures