0393
Depth-dependent functional mapping of mental prediction in human somatosensory cortex1SFIM, NIMH, Bethesda, MD, United States, 2Cognitive Neuroscience Lab, Division of Medical Bioengineering, Graduate School of Natural Science and Technology, Okayama University, Okayama, Japan
Synopsis
High-resolution, cortical depth-dependent fMRI allows researchers to address questions about the feed-forward and feed-back driven activity non-invasively. The processing of finger-touching in the sensory cortex is a complex interplay between feed-forward activity from the thalamus and feed-back input from higher-order brain areas representing touching anticipation/prediction. Here we use high-resolution (0.7mm) fMRI at 7T to investigate this interplay in the human brain with BOLD and blood-volume-sensitive fMRI as a function of cortical depth. We find that we can reveal that prediction input in S1 is dominated from superficial layers, while thalamo-cortical feed-forward input is dominant in middle cortical layers, as expected from previous work in animals.
Purpose
Cortical depth-dependent fMRI can provide information about how activity is driven by feed-forward and feed-back connections. Previous layer-dependent fMRI studies in humans focused on brain areas such as primary visual cortex [Muckli 2015], primary auditory cortex [De Martino 2015], and primary motor cortex [Huber 2017]. However, layer-dependent fMRI had not been applied in the human primary somato-sensory cortex to date. This is due to the technically challenging environment in S1: It has a cortical thickness of only 1.9 mm [Fischl and Dale 2000] – thinner than any other cortical area – and a relatively fine-scale organization [Schluppeck 2017], where individual fingers are represented in only a few voxels. In this study, we aim for the first application of layer-dependent fMRI in the primary somato-sensory cortex to investigate the mesoscopic circuitry [Felleman and Van Essen 1991][Constantinople 2013] of feed-forward driven thalamo-cortical input and feed-back driven cortico-cortical input with advanced CBV-weighted imaging methodologies at 7T. We are aiming to do so by means of custom-built MRI-compatible non-metallic tactile touching-devices [Yu 2011] and a somatosensory prediction task (Fig. 1).
Methods
Eight two-hour scan sessions were conducted at a 7T Siemens scanner with a 32-channel NOVA Medical head coil and SC72 body gradient. Data acquisition procedures were used as previously presented in [Huber 2017]. In short: We used a multi-contrast VASO-BOLD sequence with slices positioned to be approximately perpendicular to the central sulcus cortex (Fig. 2A). (Nominal in-plane resolution = 0.71mm, TI1/TI2/TR = 1.1/2.6/3.4s, twelve slices with FLASH GRAPPA-2 [Talagala 2015], matrix 44x132, 3D-EPI readout [Poser 2010], FOV 31.2x93.7x21.6mm3 (Fig. 2). Two to three 12-min functional scans were collected per participant. The four task conditions are depicted in Fig. 1B-E. For the tasks with touching in non-random order, the participants were asked to mentally predict which finger was going to be touched next. 10-min functional localizer runs were conducted to identify ROIs of the index finger in BA 3b. Segmentation and cortical layering algorithms were applied directly in the EPI functional scan space (Fig. 3A). CBV and BOLD signal changes in the index-finger region of BA3b were extracted along eleven cortical depths. For clear graphical depiction of the signal maps, smoothing (FWHM=voxel size) within the cortical layers along the cortical ribbon was applied [Blazejewska 2016] (Fig. 3F-G).
Results
The method proposed here provides sufficient anatomical contrast (Fig. 3B) and temporal stability (Fig. 3C) to identify layer-dependent activity on a single-participant basis (Fig. 3F-G). Touching in a random order showed the strongest activation in middle cortical laminae, at the presumed location of thalamic input. Mental prediction evoked additional activity in superficial cortical laminae, at the presumed location of cortico-cortical feed-back input. Cortical profiles of all task conditions are averaged across participants and are depicted in Fig. 4A. Fig. 4B confirms the single-participant results that the mental prediction is mostly arising from superficial laminae with a secondary peak in deeper laminae. Touching without mental prediction, however, is dominant in middle laminae. BOLD is more biased towards the location of pial veins compared to CBV fMRI (Fig. 4D).Discussion
Depth-dependent GE-BOLD signal is expected to be biased by large draining veins [Huber 2017], whose oxygenation might be dominated from neural activity in distant cortical layers (Fig. 4D) and in distant cortical columns of other fingers. Here, we accounted for the vascular contamination by validating the BOLD with concomitantly acquired CBV-fMRI, with superior localization specificity. The fact that the response to finger touching without prediction is highest in middle cortical layers is consistent with the animal literature of sensory tasks and suggests that it is driven by thalamo-cortical feed-forward connections (orange in Fig. 4B). The fact that the fMRI signal modulation upon mental prediction is strongest in upper cortical layers suggests that it is dominated by cortico-cortical feed-back connections (violet in Fig. 4B). For touching of finger 3, 4, 5 only, surround inhibition results in negative BOLD signal (brown in Fig. 4C) in the index finger regions of BA3b. The surround inhibition results in the largest BOLD signal change in middle cortical layers while CBV-decrease is dominant in upper layers. This layer-dependent uncoupling between BOLD and CBV-fMRI is consistent with previous surround-inhibition results in the visual cortex of monkeys [Goense 2012] and humans [Huber 2014].Conclusion
Here, we presented the first application in humans of layer-dependent fMRI in the technically challenging area of primary sensory cortex. We were able to identify layer-dependent features of different feed-forward and feed-back activity that is consistent with the micro-circuitry expected from animal studies. We confirmed that CBV-based fMRI has a higher localization specificity than BOLD and provides results with improved interpretability.Acknowledgements
YY is supported by Grant-in-Aid for JSPS Research Fellow (17J40084) from the Japan Society for the Promotion of Science (JSPS).
JY is supported by Grant-in-Aid for Challenging Research (17K18855) from the Japan Society for the Promotion of Science (JSPS).
This research is supported by the NIMH Intramural Research Program (#ZIA-MH002783). We thank the NIH machine shop for support in 3d-printing the piano-key touching device. We thank Daniel Glen for advice in high-quality data registration. The study was approved under NIH Combined Neuroscience Institutional Review Board protocol #93-M-0170 (ClinicalTrials.gov identifier: NCT00001360).
We thank Dimo Ivanov for proof reading the abstract. We thank Dimov Ivanov and Benedikt Poser for their contribution to the MR-sequence code.
References
[Blazejewska 2016] Blazejewska et al., 2016, OHBM, #1728, Improved tSNR of high-resolution fMRI with surface-based cortical ribbon smoothing;
[Constantinople 2013] Constantinople et al., 2013, Science, 340: 1591-1594. Deep cortical layers are activated directly by Thalamus;
[De Martino 2015] De Martino et al., 2015, PNAS, 112: 16036–16041. Frequency preference and attention effects across cortical depths in the human primary auditory cortex.
[Felleman and Van Essen 1991] Felleman and van Essen, 1991, Cerebr. Cortex, 1:1-47, Distributed hierarchical processing in the primate cerebral cortex.
[Fischl and Dale 2000] Fischl and Dale, PNAS, 2000, 97:11050-11055, Measuring the thickness of the human cerebral cortex from magnetic resonance images
[Goense 2012] Goense et al., 2012, 76: 629-639 Neuron, High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses
[Graziano 2016] Graziano, 2016, Cell, 20:121-132, Ethological Action Maps: A Paradigm Shift for the Motor Cortex
[Huber 2014a] Huber et al., 2014, 97: 349-362, NeuroImage, Investigation of the neurovascular coupling in positive and negative BOLD responses in human brain at 7T
[Huber 2014] Huber et al., 2014, MRM 72:137-148, Slab-selective, BOLD-corrected VASO at 7 tesla provides measures of cerebral blood volume reactivity with high signal-to-noise ratio.
[Huber 2017] Huber et al., 2017, Neuron, accepted: in print, pii S0896-6273(17)31033-4, High-resolution CBV-fMRI allows mapping of laminar activity and connectivity of cortical input and output in human M1.
[Muckli 2015] Muckli et al., 2015 Current Biology, 25: 2690-2695 Contextual Feedback to Superficial Layers of V1.
[Poser 2010] Poser et al., 2010, NeuroImage, 51: 261-266, Three dimensional echo-planar imaging at 7 tesla
[Schluppeck 2017] Schuppleck et al., 2017, NeuroImage, ahead of print: 10.1016/j.neuroimage.2017.01.081, Exploring structure and function of sensory cortex with 7 T MRI
[Talagala 2015] Talagala et al., MRM, 2015, 75:2362-2371, Improvement of temporal signal-to-noise ratio of GRAPPA accelerated echo planar imaging using a FLASH based calibration scan
[Shi 2017] Shi et al., 2017, PNAS, 20: 5253–5258. High spatial correspondence at a columnar level between activation and resting state fMRI signals and local field potentials
[Yu 2011] Yu et al., IEEE/ICME, 2011, 526-531, Development and evaluation of a MRI-compatible tactile orientation stimulator
Figures
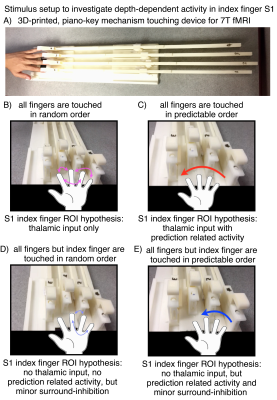
Fig. 1: Stimulation setup for the application at 7 Tesla.
A) The touching device is custom-designed, consists of 3d-printed plastic, and is completely metal free. Its design is inspired by the piano-key mechanism. It is manually operated inside the scanner room. The length is determined by the long bore of the 7T magnet size. B-D) depict the different touching sequences. B) Touching of fingers 2, 3, 4, 5 in random order. C) Touching of fingers 2, 3, 4, 5 in predictable order. D) Touching of fingers 3, 4, 5 in random order, E) Touching of fingers 3, 4, 5 in predictable order.
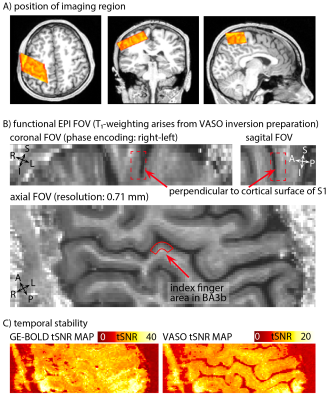
Fig. 2: Features of the EPI data: Orientation, anatomical contrast, and temporal stability of one representative participant.
A) depicts the FOV of the functional imaging region, overlaid on a whole-brain anatomical T1-weighted dataset (from 3T). B) depicts the mean VASO signal of one representative twelve-min functional scan. The FOV is positioned to be perpendicular to the index finger region of BA3b, allowing us to use a coarser slice-thickness (1.8 mm) compared to in-plane resolution (0.71mm). C) depicts representative tSNR maps of BOLD and VASO. Note the different color scale.
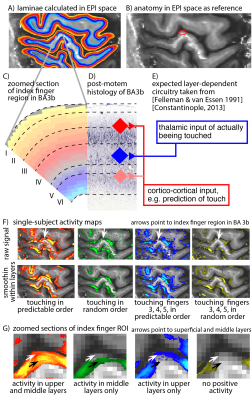
Fig. 3:
A) Layers are identified directly in EPI space. This avoids the risk of errors in registration, distortion correction, or resolution loss due to spatial resampling. B) expected layer-dependent circuitry following [Felleman & van Essen 1991] and [Constantinople 2013]. Thalamo-cortical feed-forward input into S1 is expected in middle layer IV, while most of the cortico-cortical feedback input is expected in superficial layers II/III. Panels F-G) depict activity maps with and without smoothing along the cortical ribbon. No smoothing was applied across laminae. It can be seen that the four tasks have a different distribution of activity across laminae.
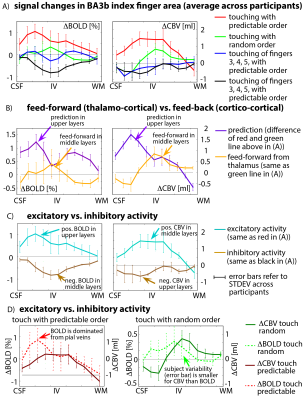
Fig. 4: cortical profiles of BOLD and VASO activity changes in ROI of index finger in BA3b, averaged across participants.
A) The four tasks result in markedly different cortical activity profiles - they are not scaled versions of each other. B) Feed-back modulated activity is dominant in superficial laminae (presumably layers II/III), while feed-forward activity is strongest in middle laminae (presumably layer IV). C) Negative BOLD is larger in middle/deeper layers, while negative CBV activity is more shifted towards superficial layers compared to their positive counterparts. D) BOLD appears more influenced by pial veins than CBV and shows higher inter-participant variability.