0387
An Optimized 3D Stack-of-Stars TSE Pulse Sequence for Simultaneous T2-weighted Imaging and T2 Mapping1Electrical and Computer Engineering, University of Arizona, Tucson, AZ, United States, 2Medical Imaging, University of Arizona, Tucson, AZ, United States, 3Siemens Healthcare, Tucson, AZ, United States, 4Department of Surgery, University of Arizona, Tucson, AZ, United States, 5Biomedical Engineering, University of Arizona, Tucson, AZ, United States
Synopsis
A 3D stack-of-stars Turbo Spin Echo sequence is presented for efficient T2-weighted imaging and T2 mapping. The pulse sequence parameters are optimized for T2 estimation, SNR, and SAR and the view ordering is designed to enable efficient k-space coverage for both non-selective and slab selective acquisitions. The technique provides excellent anatomical coverage within clinically acceptable times. Utility of the sequence is demonstrated in vivo on the knee, brain and for carotid vessel wall imaging.
Introduction
3D turbo spin echo (TSE) sequences have been used for isotropic T1- and T2-weighted imaging1-3. These sequences typically use a Cartesian trajectory and employ very long echo train lengths, optionally with variable refocusing flip angles to achieve the desired contrast. Quantitative T2 mapping using 3D TSE has suffered from long acquisition times, due to the need to acquire data at different echo times (TE). While alternate approaches4,5 have been proposed for time efficient acquisition of images at multiple TEs, they are based on sampling a rectilinear k-space grid. Recently, a stack-of-stars TSE sequence with empirically chosen variable refocusing flip angles was proposed for motion robust T2 mapping and T2-weighted imaging6. The sequence used a radial trajectory to sample each slice, with Cartesian Fourier encoding along the third dimension. However, the sequence was limited to non-selective excitation and suffered from long scan times. In this work, we present an efficient stack-of-stars TSE pulse sequence with (i) refocusing flip angles designed based on an analytical framework and (ii) a view ordering optimized for selective and non-selective 3D imaging. The sequence is evaluated in vivo on the knee, brain, and for carotid imaging.Methods
Pulse Sequence:
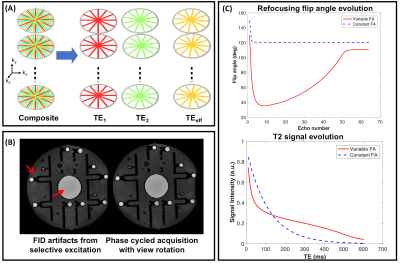
A Cartesian 3D TSE pulse sequence was modified to sample k-space in a stack-of-stars trajectory and the view ordering was chosen to acquire under-sampled k-space data at each TE (Figure 1A). Since the same set of angles are acquired in each kz plane, parallel imaging along the slice dimension was incorporated to further accelerate the acquisition. In order to suppress the free induction decay (FID) signal generated from outside the excited slab, crusher gradients were added around the refocusing RF pulses7. For selective 3D imaging, the use of non-selective refocusing results in FID artifacts which cannot be cancelled by crusher gradients8,9. Cartesian sequences address these artifacts by phase cycling over two measurements and averaging the k-space lines prior to reconstruction9. Because the center of k-space for each kz plane is densely sampled compared to the outer parts in a radial acquisition, the acquired radial views can be rotated across the phase cycled measurements. This allows better coverage of k-space while also ensuring cancellation of the artifacts (Figure 1B).
Flip Angle Design:
The refocusing flip angle scheme was designed to: (i) minimize the T2 estimation error, (ii) minimize B1+rms, and (iii) optimize SNR by maximizing the area under the T2 decay curve. The flip angles were computed using a prospective echo phase graph (EPG) algorithm10, parametrized by four control angles $$$\vec{\alpha} = [\alpha_{min},\alpha_{cent},\alpha_{end},\alpha_{max}]$$$. A Cramer-Rao lower bound (CRLB) analysis was performed to understand the effect of the control angles on the estimated T2 values. The CRLB analysis resulted in a reduced search space for $$$\vec{\alpha}$$$. The signal evolution corresponding to the optimized flip angle scheme is shown in Figure 1C.
In vivo Imaging:
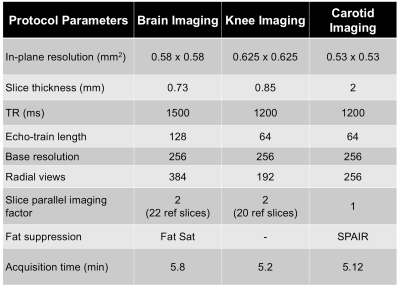
The pulse sequence was implemented on a 3T Skyra Siemens platform and subjects were imaged after obtaining informed consent in agreement with the institutional review board requirements. In vivo data were acquired using the proposed sequence for both slab selective and non-selective excitation. The sequence parameters are shown in Table 1.
All data were reconstructed offline using a subspace constrained algorithm6,11. The reconstruction generated images at different TEs from which T2 maps were obtained by fitting them to a library of signal decay curves.
Results
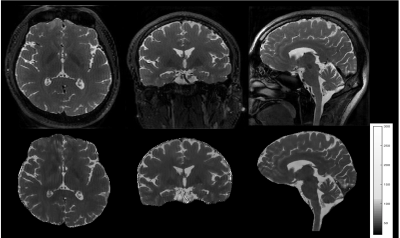
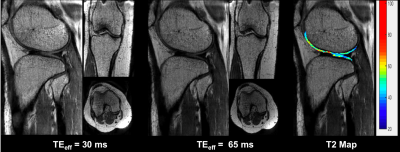
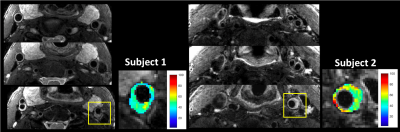
Reformatted T2-weighted images and T2 maps of the brain from a volunteer are shown in Figure 2. 192 sagittal slices were acquired with 0.7mm slice thickness and 0.58mm in-plane resolution to cover the whole brain in 5.8 minutes. Figure 3 shows images of the knee at two different echo times along with a T2 map of the cartilage overlaid on a sagittal slice. Use of the slab selective variant of the pulse sequence for carotid vessel wall imaging is demonstrated in Figure 4. Data were acquired in two patients using a dedicated carotid coil12 with 2 mm thick slices and 0.5mm in-plane resolution. T2 values of the left carotid wall are consistent with values reported in plaques. The proposed pulse sequence enables the acquisition of TE images and T2 maps with excellent coverage of the anatomy within a 5-minute scan.Conclusion
A radial stack-of-stars TSE pulse sequence has been presented for simultaneous T2-weighted 3D imaging and T2 mapping. The pulse sequence is optimized for parameter estimation, SNR, and SAR. The technique provides excellent anatomical coverage within clinically acceptable times. Utility of the sequence has been demonstrated in vivo for both non-selective and slab selective imaging applications.Acknowledgements
The authors would like to acknowledge support from the Arizona Biomedical Research Commission (Grant ADHS14-082996) and the Technology and Research Initiative Fund (TRIF) Improving Health Initiative.References
1. Kijowski R, Davis KW, Woods MA, et al. Knee Joint: Comprehensive Assessment with 3D Isotropic Resolution Fast Spin-Echo MR Imaging—Diagnostic Performance Compared with That of Conventional MR Imaging at 3.0 T. Radiology 2009;252:486-95
2. Li CQ, Chen W, Rosenberg JK, et al. Optimizing Isotropic 3D FSE Methods for Imaging the Knee. J Magn Reson Imaging 2014;39:1417-25
3. Lee S, Jee W-H, Jung J-Y, et al. MRI of the lumbar spine: comparison of 3D isotropic turbo spin-echo SPACE sequence versus conventional 2D sequences at 3.0 T. Acta Radiol 2015;56:174–181
4. Tamir, J. I., Uecker, M., Chen, W., Lai, P., Alley, M. T., Vasanawala, S. S. and Lustig, M., T2 shuffling: Sharp, multicontrast, volumetric fast spin-echo imaging. Magn. Reson. Med. 2017, 77: 180–195.
5. Tamir JI, Taviani V, Vasanawala SS, and Lustig M, T1-T2 Shuffling: Multi-Contrast 3D Fast Spin-Echo with T1 and T2 Sensitivity. Proc ISMRM 2017.
6. Keerthivasan, M Saranathan, A Bilgin, DR Martin, MI Altbach, High-resolution 3D T2 mapping using a stack-of-stars radial FSE pulse sequence. Proc. ISMRM 2017.
7. Han M, Chiba K, Banerjee S, Carballido-Gamio J, Krug R. Variable Flip Angle 3D Fast Spin-Echo Sequence Combined with Outer Volume Suppression for Imaging Trabecular Bone Structure of the Proximal Femur. Journal of magnetic resonance imaging : JMRI. 2015;41(5):1300-1310.
8. Mugler JP 3rd. Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Imaging 2014;39:745–67
9. Magland JF, Rajapakse CS, Wright AC, Acciavatti R, Wehrli FW. 3D Fast Spin Echo With Out-of-Slab Cancellation: A Technique for High-Resolution Structural Imaging of Trabecular Bone at 7 Tesla. Magnetic resonance in medicine. 2010;63(3):719-727.
10. Busse RF, Brau A, Vu A, et al. Effects of refocusing flip angle modulation and view ordering in 3D fast spin echo. Magn Reson Med 2008;60:640–49
11. Huang C., Bilgin A., Barr T., Altbach M, T2 relaxometry with indirect echo compensation from highly undersampled data, Magnetic Resonance in Medicine, 70(4) 2013.
12. Beck, M. J., Parker, D. L., Bolster, B. D., Kim, S.-E., McNally, J. S., Treiman, G. S. and Hadley, J. R., Interchangeable neck shape–specific coils for a clinically realizable anterior neck phased array system. Magn. Reson. Med. 2017. doi:10.1002/mrm.26632
Figures