0385
Interleaved SPEN with per-shot correction for high definition human DTI measurements1Department of Chemical and Biological Physics, Weizmann Institute of Science, Rehovot, Israel
Synopsis
Sub-mm DTI is of great interest for clinical diagnosis and healthy tissue studies. Segmented imaging is hindered in these applications, due to complications associated to unavoidable motions in-between shots. This work demonstrates the advantages resulting from using interleaved, segmented SPatially ENcoded (SPEN) methods for DTI, thanks to their ability to (i) provide low-resolution but fully sampled images per shot; (ii) compensate for rigid-body motions by simple phase corrections, and (iii) zoom on restricted FOVs without folding complications. All this enables high-resolution diffusion MRI on humans, as shown here by delineating the pons anatomy at a 0.74mm in-plane resolution.
INTRODUCTION
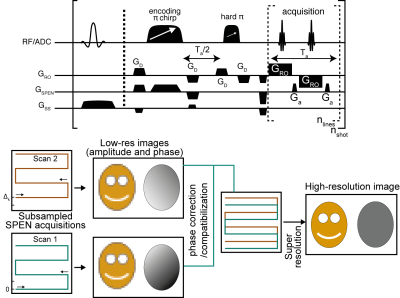
Diffusion imaging is of major radiological interest,1 with applications including tumor detection,2 stroke characterizations3 and connectomics.4 Thanks to their independence from deleterious inter-scan motion effects, single-shot techniques like EPI are usually needed to map the µm-size motions being sought in quantitative diffusion measurements. While resolution and robustness to inhomogeneities are limited in single-shot EPI, multi-shot interleaving can be used for improving these images’ quality. In diffusion measurements, however, each shot will be impacted by spatially-dependent phases, arising from interferences between collective motions and the diffusion sensitizing gradients. Unless suitably accounted for, e.g. using parallel-imaging or navigators,5 these effects will hinder the reconstruction of quantitative diffusion images. Techniques that rescan the center of the k-space in each shot and deduce the phase changes from redundant information, are often needed to avoid these effects.6 SPatiotemporal ENcoding (SPEN) is a single-shot MRI technique which excites the spins and rasterizes the image profile in a spatially sequential manner, rather that acquiring signals of all spins simultaneously in the k-domain.7 SPEN’s resolution and FOV are defined at excitation rather than at acquisition, allowing one to use stronger phase-encoding gradients than in EPI. This imparts higher robustness against inhomogeneity-induced distortions, and prevents folding artifacts even upon zooming along the “low-bandwidth” (SPEN) axis. Furthermore, by contrast to what occurs in interleaved EPI, subsampling this axis will not bring about image folding effects: it will only lead to lower resolution SPEN images, which can be co-processed for improving resolution as per the scheme in Figure 1. In this work, we exploit this to implement sub-mm multi-shot diffusion weighted human imaging of challenging human regions.
METHODS
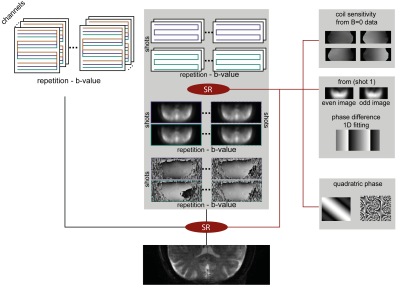
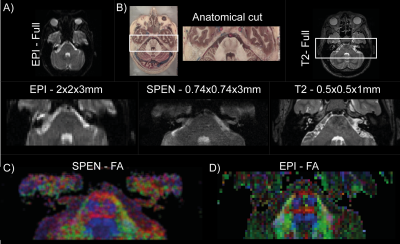
A fully-refocused multi-shot SPEN sequence to achieve high-resolution DTI was written for our Siemens TrioTIM 3T scanner, on the basis of the scheme in Figure 1. The acquisition scheme was modeled on the interleaved procedure of Schmidt et al,8 but endowed with a new reconstruction scheme. In this each separate shot is first reconstructed as two separate images, one for the even- and the other for the odd-readout data set, producing unique low definition –but folding-free– images and phase maps; these are compatibilized, and merged together. This low-definition reconstruction is then repeated for each interleaved shot, for each diffusion direction, and eventually for multiple signal-averaging repetitions, independently. The final reconstruction scheme then incorporates each coil’s sensitivity map (obtained from the b0 SPEN data, without additional calibrations, using ESPiRIT9), the specific phase map of the separately acquired shots, and new SPEN Super-resolution (SR)10 algorithm incorporating L2 finite-difference regularization11 (Figure 2). From these data, high-definition ADC/DTI maps were computed by accounting the effects of both the imaging and the diffusion gradients, along the lines described in Refs.12,13. All MRI-experiments were recorded using a 4-channel head coil, on human volunteers after signing of suitable consents. Interleaved SPEN diffusivity images were acquired using 4 interleaved shots for a resolution of 0.74x0.74x3mm, using 12 diffusion direction and a nominal b=750 s/mm2. For comparison, the maximum definition that could be obtained in single-shot EPI DTI experiments on the same region was 2x2x3mm.RESULTS & DISCUSSION
Experiments on the pons, a central brain region ca. 8 cm in length, were recorded using the protocol just described. A summary of the achieved results is shown in Figure 3. These provided similar ADC maps as EPI counterparts (not shown). However, SPEN provided clearly higher details about the fibers inside the pons region, enabling the delineation of the pyramidal tract, the oculomotor nerve (CNIII, visible in blue), and the decussation of the medial lemniscus that are lost in EPI’s images.CONCLUSION
The capacity to reconstruct quantitative diffusivity maps from high-resolution, multi-shot, signal averaged SPEN experiments has been demonstrated. These images are achieved under conditions which challenge state-of-the-art EPI, thanks to SPEN’s image- (as opposed to k-space) based nature.Acknowledgements
We are grateful to Dr. Sagit Shushan (Wolfson MC) and Edna Furman-Haran (Weizmann) for assistance. SfC thanks the Feinberg Graduate School (Weizmann) and French Foreign Service for partial postdoctoral fellowships. Support came from the Israel Science Foundation (#2508/17), the EU ERC-2016-PoC grant # 751106, the Minerva foundation (#712277), the Kimmel Institute for Magnetic Resonance and the generosity of the Perlman Family Foundation.References
1. Mori, S., Zhang, J., 2006. Principles of Diffusion Tensor Imaging and Its Applications to Basic Neuroscience Research. Neuron; 51, 527–539.
2. Assaf, Y., Pasternak, O., 2008. Diffusion Tensor Imaging (DTI)-based White Matter Mapping in Brain Research: A Review. J. Mol. Neurosci; 34, 51–61.
3. Werring, D. J., Toosy, A. T., Clark, C. A., Parker, G. J., Barker, G. J., Miller, D. H., & Thompson, A. J. (2000). Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J. Neurol. Neurosurg. Psychiatry; 69(2), 269-272.
4. Beaulieu, C., 2002. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed; 15, 435–455.
5. Zhang, Z., Huang, F., Ma, X., Xie, S., Guo, H., 2015. Self-feeding MUSE: A robust method for high resolution diffusion imaging using interleaved EPI. NeuroImage 105, 552–560.
6. Pipe, J. G., Farthing, V. G., & Forbes, K. P. (2002). Multishot diffusion‐weighted FSE using PROPELLER MRI. Magn. Reson. Med.; 47(1), 42-52.
7. Tal, A., & Frydman, L. (2010). Single-scan multidimensional magnetic resonance. Prog Nucl Magn Reson Spectrosc; 57(3), 241-292.
8. Schmidt, R., Seginer, A., Frydman, L., 2016. Interleaved multishot imaging by spatiotemporal encoding: A fast, self-referenced method for high-definition diffusion and functional MRI: Referenceless Interleaved SPEN MRI. Magn. Reson. Med.; 75, 1935–1948.
9. Uecker, M., Lai, P., Murphy, M.J., Virtue, P., Elad, M., Pauly, J.M., Vasanawala, S.S., Lustig, M., 2014. ESPIRiT-an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn. Reson. Med. 71, 990–1001.
10. Ben-Eliezer, N., Irani, M., Frydman, L., 2010. Super-resolved spatially encoded single-scan 2D MRI. Magn. Reson. Med.; 63, 1594–1600.
11. Lustig, M., Donoho, D.L., Santos, J.M., Pauly, J.M., 2008. Compressed Sensing MRI. IEEE Signal Process.; 25, 72–82.
12. Solomon, E., Liberman, G., Nissan, N., & Frydman, L. (2017). Robust diffusion tensor imaging by spatiotemporal encoding: Principles and in vivo demonstrations. Magn. Reson. Med.; 77(3), 1124-1133.
13. Solomon, E., Nissan, N., Furman-Haran, E., Seginer, A., Shapiro-Feinberg, M., Degani, H., Frydman, L., n.d. Overcoming limitations in diffusion‐weighted MRI of breast by spatio-temporal encoding. Magn. Reson. Med.; 73, 2163–2173.
14. Ben-Eliezer, N., Irani, M., Frydman, L., 2010. Super-resolved spatially encoded single-scan 2D MRI. Magn. Reson. Med.; 63, 1594–1600.
15. Jellison, B. J., Field, A. S., Medow, J., Lazar, M., Salamat, M. S., & Alexander, A. L. (2004). Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. Am J Neuroradiol; 25(3), 356-369.
Figures