0384
Synthetic MP2RAGE: multiple ‘on-demand’ contrasts from a single acquisition1Aix-Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 3Siemens Healthcare SAS, Saint-Denis, France, 4Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 5Department of Radiology, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland, 6Signal Processing Laboratory (LTS 5), École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Synopsis
MP2RAGE and FLAWS sequences are increasingly used for brain clinical research imaging at ultra-high field. Yet, the ability to provide at the same time an optimal contrast between GM and WM for segmentation, an accurate T1 mapping and/or the ability to highlight only a single tissue or lesions, is in practice not possible with only one single MP2RAGE acquisition. In this work, we demonstrate that synthetic ‘uniform’ images with ‘on-demand’ clinically relevant contrasts could be generated at 7T from a single MP2RAGE acquisition providing an accurate T1 map, allowing for instance tissue signal nulling or lesion signal enhancement.
Introduction
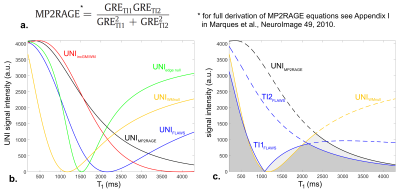
The magnetization-prepared two rapid acquisition gradient echoes (MP2RAGE1) is an extension of the MPRAGE sequence acquiring two GRE volumes at different inversion times. These volumes can be combined to generate a uniform T1-weighted volume (UNI) with strongly reduced influence1 of T2*, B1- and M0. By integrating Bloch equations, a T1 map can also be computed from this UNI volume. Consequently, the MP2RAGE has been shown to be superior to MPRAGE, providing better automated tissue segmentation as well as fast and robust whole-brain T1 mapping1. Currently, several studies performed at 7T also acquired a B1+ map to correct for bias in the T1 estimation2,3.
On the other hand, fluid and white matter suppression (FLAWS4), an MP2RAGE with specific inversion times, was introduced to enable better cortex visualization by taking the minimum pixel intensity of the two volumes to suppress both WM and cerebrospinal fluid (CSF) signals. Both these sequences have been demonstrated well suited to multiple sclerosis (MS) and epilepsy lesions imaging5,6. Altogether, running a single MP2RAGE acquisition in order to obtain optimal GM/WM contrast, accurate T1 mapping and FLAWS contrast, all with minimal B1+ bias, would be ideal but is practically not feasible.
In this work, we therefore propose to build multiple synthetic UNI images with ‘on-demand’ contrasts based on a single robust MP2RAGE T1 map acquisition by re-integrating Bloch equations. The resulting synthetic volumes could facilitate lesion visualization in pathological brain tissue or GM/WM automated segmentation, while simplifying MP2RAGE parameters choice.
Methods
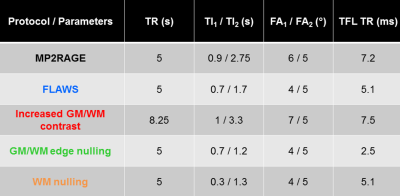
One healthy volunteer and two patients, suffering from MS and epilepsy respectively, were scanned using an investigative 7T MR system (Siemens Healthcare, Erlangen, Germany) and a 1Tx/32Rx head coil (Nova Medical). B1+ maps were acquired with a magnetization-prepared turbo-FLASH sequence7. Whole brain 3D sagittal MP2RAGE acquisitions were performed with a (0.6 mm)3 isotropic resolution (FOV: 240 mm, slices: 256). MP2RAGE parameters for the real and synthetic protocols are described in Figure 1. B1+ bias correction was performed offline using a look-up table built on MATLAB2,3. Synthetic UNI volumes with ‘on-demand’ contrasts were generated with MATLAB by varying MP2RAGE specific parameters empirically (Figure 1-2) and re-injecting T1 values into Bloch equations (Figure 2).Results
Relationships between T1 values and UNI signals given by the Bloch equations for all real and synthetic protocols are shown in Figure 2. Depending on sequence parameters, the signal from specific T1-ranges may be nulled, compressed, or amplified to modify the image contrast.
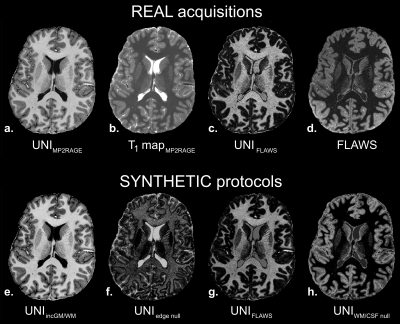
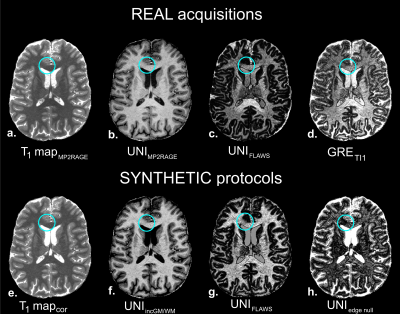
Resulting images on the healthy volunteer are illustrated in Figure 3. A synthetic UNIFLAWS volume (3.g) could be generated as the proof of concept of synthetic framework, using FLAWS parameters and the B1+-corrected T1 map (3.b) of the MP2RAGE acquisition (3.a). Qualitatively, this synthetic volume showed very similar contrast (i.e. GM nulling) as compared to the real UNIFLAWS of the FLAWS acquisition (3.c). Additional ‘on-demand’ UNI volumes could also be created using synthetic protocols such as: increased GM/WM contrast (3.e), GM/WM edge nulling contrast (3.f) and WM/CSF nulling contrast (3.h). The latest exhibits hyper-intense cortex signal, similarly to the acquired FLAWS volume (3.d), with additional removal of B1 bias.
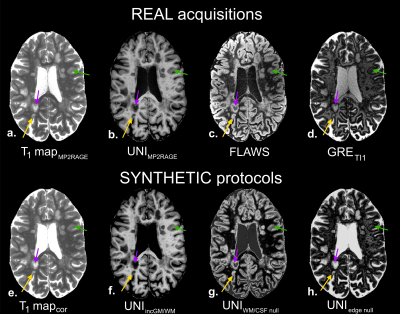
Resulting images on the two patients are shown in Figures 4 and 5. For both patients, synthetic images were generated from the corrected T1 maps of the real MP2RAGE acquisitions, and were corrected from B1 bias. MS lesion visualization (subcortical, cortical and within WM) were improved using UNIincGM/WM, UNIWMCSFnull and UNIedgenull contrasts, when compared to their originally acquired counterparts (UNIMP2RAGE, FLAWS and GRETI1). In epilepsy, focal cortical dysplasia in the corpus callosum, associated to abnormal T1 value range, exhibited hypo-signal in UNIFLAWS and UNIedgenull.
Discussion
By relying on the unbiased T1 map, synthetic UNI volumes were almost devoid of any bias, which enabled uniform, purely T1-based signal and contrast manipulation. Using this synthetic framework, tissue contrast could be non-linearly modulated and fully controllable by tuning the MP2RAGE parameters. Synthetic UNI images could thus be seen as T1 ‘filters’ which enhanced desired T1 dynamics. With synthetic MP2RAGE, tissue automated segmentation, as well as lesion enhancement for pathological brains, have the potential to be improved to some extent. These improvements could likely facilitate clinical interpretations. Interestingly, this synthetic framework could also allow saving the duration of a high-resolution FLAWS acquisition (about 12 min), which is an important advantage to consider for protocol design. In the future, this work could be transposed to the spinal cord, which possesses very different T1 values for GM3 as compared to the brain, hence offering new tools to study degenerative pathologies.Acknowledgements
The authors would like to thank Véronique Gimenez and Lauriane Pini for the study logistics. This work was supported by the following funding sources: Investissements d’Avenir 7T-AMI-ANR-11-EQPX-0001, A*MIDEX-EI-13-07-130115-08.38-7T-AMISTART, A*MIDEXANR-11-IDEX-0001-02, Aix-Marseille Université, AP-HM and CNRS (Centre National de la Recherche Scientifique).References
[1] Marques et al., NeuroImage 49, 2010. [2] Marques and Gruetter, PLoS One 8, 2013. [3] Massire et al., NeuroImage 143, 2016. [4] Tanner et al., JMRI 35, 2012. [5] Kober et al., Invest. Radiol. 47, 2012. [6] Obusez et al., NeuroImage 2016. [7] Fautz et al., ISMRM 2008, p 1247.Figures