0383
Highspeed Imaging with Sub-millisecond Temporal Resolution of the Vocal Folds Oscillations using EGG-Gated Gradient Echo with Rapid Phase Encoding1Dept.of Radiology, Medical Physics, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2German Consortium for Translational Cancer Research Freiburg Site, German Cancer Research Center (DKFZ), Heidelberg, Germany, 3Institute of Musicians' Medicine, Freiburg University Medical Center, Germany Faculty of Medicine, University of Freiburg, Freiburg, Germany
Synopsis
We present a novel encoding method that allows sampling of one-dimensional periodic motion with sub-millisecond time resolution by applying very short phase encoding gradients along the direction of motion. The technique is applied to the oscillatory motion of the vocal folds during singing, and MR data are gated using an electroglottography synchronisation signal. Dynamic images during vocal fold oscillation were acquired with a temporal resolution of about 690 µs, and closing of the vocal folds was assessed.
Introduction
State-of-the-art retrospectively-gated imaging of physiologic movement (e.g., cardiac motion) can readily achieve a temporal resolution of 10-20 ms, but is inherently limited by the readout duration. In this work we demonstrate that it is possible to measure periodic motion with much higher temporal resolution by using very short phase encoding gradients and a simultaneously-acquired signal for synchronization with the motion. Here, an electroglottography (EGG) signal is used to image the oscillatory motion of the vocal folds during singing in one exhalation with sub-millisecond temporal resolution.Materials and Methods
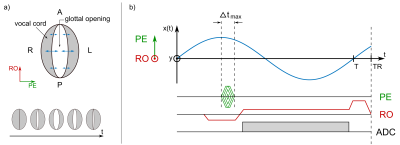
During sound creation (phonation), the vocal folds perform a lateral oscillatory motion in LR direction (Fig. 1a) with frequencies in the acoustic range (about 60-1500 Hz). To image this rapid motion with $$$N$$$ time-frames per cycle duration $$$T = 1/f$$$, even for a low singing frequency of $$$f = 200$$$ Hz a temporal resolution of $$$\Delta T = T/N = 500$$$ µs is required at $$$N = 10$$$ frames. This high temporal resolution is only possible in phase encoding (PE) direction, if the duration of the gradient is chosen as short as possible (Fig. 1b) – this can be achieved by a gradient table with increasing duration for outer k-space lines. The AP direction was chosen as the readout (RO) direction as motion of the vocal folds is negligible in this direction.
For sub-millisecond vocal fold imaging a conventional spoiled gradient echo sequence (FLASH) was modified using a time-optimal PE gradient lobe for every k-space line (Fig. 1b). Images of the vocal folds were acquired in a transverse slice orientation using the following parameters: TE = 3.47 ms, TR = 7.4 ms, BW/Px = 240Hz, FoV = 70x70 mm2, imaging matrix 80x80. Partial Fourier of 6/8 and asymmetric echo of 40/60 were employed to further reduce acquisition time per image. On a clinical 1.5 T MRI system (Tim Symphony; SIEMENS Healthineers, Erlangen, Germany) with a 30 mT/m gradient system a PE duration of $$$\Delta t_\mathrm{max} = 750$$$ µs could be achieved for the outermost k-space line. At an acquisition time per image of 0.44 s, 57 averages were acquired to achieve a total measurement time of 25 s, which is about the longest time for volunteers to sing a constant tone. The large amount of averages ensures that every k-space line is sampled at least once for every phase of the oscillatory motion.
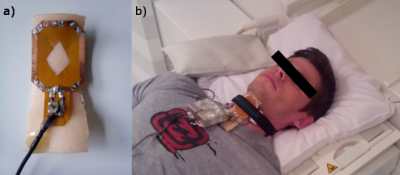
A modified, MR-safe EGG unit (EGG-D400, Laryngograph Ltd., London, UK) was used to measure the time dependent opening and closing of the vocal folds during the measurement1. MR data was acquired with a custom-built Tx/Rx coil that was fixed to the neck of the volunteer at the position of the larynx, between the EGG electrodes (Fig. 3). An optical trigger in the MR sequence was coupled into the EGG unit to synchronize between EGG and MR data.
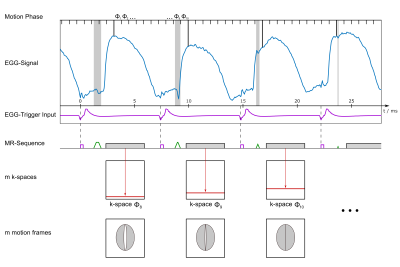
To assign the correct motion phase $$$\phi_m\ (m=1\ldots N)$$$ to each acquired k-space line $$$k_n$$$, the position of the optical trigger is identified in the EGG data and a sine wave is fitted to the EGG curve to extrapolate the correct phase of the oscillatory motion at the time of the PE gradient. The lines are then sorted into $$$N=10$$$ phases, and after applying a Hanning-Filter, a Fourier transform is performed for image reconstruction (Fig. 3).
Results and Discussion
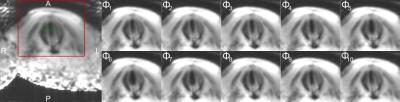
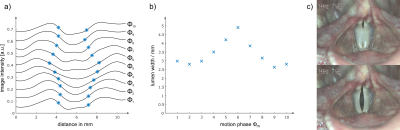
Figure 4 shows the oscillatory motion of the vocal folds at a singing frequency of $$$f = 145$$$ Hz resolved in 10 contiguous phases ($$$\Delta T \approx 690$$$ µs). The periodic opening of the vocal folds can clearly be observed, and opening widths between 2.6 and 4.9 mm were measured (Fig. 5a,b). The MR images show a small open lumen throughout the oscillation, whereas laryngeal stroboscopy shows a full closing of the vocal folds under phonation (Fig. 5c). This discrepancy is attributed to partial volume effects from tissue below the surface of the vocal folds (e.g., musculus vocalis) or susceptibility artefacts. Nevertheless, the rapid motion of the vocal folds could be imaged with MR for the first time.
Temporal resolution and image quality can further be improved using higher field strengths and stronger gradient systems. Other repetitive motion patterns such as the opening and closing of heart valves could be assessed using an ECG signal for synchronization.
Conclusion
For the first time, MR images with sub-millisecond time-resolution are reconstructed with retrospective EGG-gating of the vocal fold oscillation, so that the fast oscillatory motion can be studied. This method opens new diagnostic options for the analysis of diseases associated with vocal fold abnormalities.Acknowledgements
No acknowledgement found.References
1 Özen AC, Traser L , Echternach M, Dadakova T, Burdumy M, Richter B, Bock M. (2016), Ensuring safety and functionality of electroglottography measurements during dynamic pulmonary MRI. Magn Reson Med. 2016 Nov;76(5):1629-1635.Figures