0380
Single-scan multi-spin-echo SPEN for dynamic T2 mapping and for 3D T2 weighted anatomical imaging1Department of Chemical and Biological Physics, Weizmann Institute of Science, Rehovot, Israel
Synopsis
SPatiotemporal ENcoding (SPEN) provides single-shot 2D images free from T2* effects, and with enhanced robustness to field distortions. SPEN’s acquisition module is relatively short, opening the possibility to combine SPEN with a multi-spin-echo protocol, thus collecting several images in one shot. This work explores this possibility towards two different aims: the single-shot characterization of spatially-resolved T2 maps, and the accelerated acquisition of 3D images incorporating a phase encoding. Both approaches proved successful, as exemplified with real-time T2 mapping of in vivo kidney on a perfused mouse and high resolution volumetric acquisitions on ex vivo phantoms and human volunteers.
INTRODUCTION
T2, T2* and quantitative susceptibility mapping are valuable techniques [1-4], but their execution may involve long scan durations, and thereby be affected by motion- or flow-induced artifacts. SPatio-temporal ENcoding (SPEN) is a single-shot technique that has been introduced as a complement to EPI, and which could shorten these experiments [5]. Thanks to its reliance on a direct spatial acquisition that is free from traditional Nyquist criteria, SPEN enables the use of strong gradients along the low-bandwidth dimension. This can help overcome image distortions arising from B0-or shift heterogeneities [6]; it can also produce improved an image’s resolution by relying on restricted FOVs free from aliasing artifacts. The relatively short sampling duration of SPEN experiments, usually shorter than T2, opens the possibility of combining these advantages with the incorporation of an additional modulating variable, by means of Multi-Spin-Echo (MSE) approaches. This study presents the advantages resulting from doing so in both preclinical and clinical settings. Specific opportunities that are here discussed include (i) quantitative single-shot T2 mappings of phantoms and of a human brain; (ii) the real-time monitoring of T2 changes upon injection of contrast material into live mice, and (iii) the possibility to use the MSEs for phase-encoding a slab-selection axis (z) to accelerate volumetric SPEN acquisitions with 3D isotropic resolution.METHODS
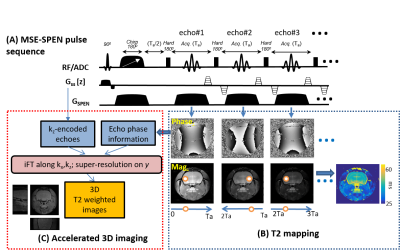
Phantom and rodents experiments were performed on an Agilent DD2® 7T/110mm scanner using a quadrature probe; human scans were acquired at 3T using a Siemens TrioTIM® scanner and a 12-channel head coil following suitable written consents. The MSE SPEN sequence used in this study is shown in Figure 1A, in a version where the spin-echoes are interspersed with optional gradient blips along the slab-selecting (z) direction. Should these gradients be absent, the sequence would still reflect in the magnitude of each echo the T2 decay (orange points in Fig.1B) –but not the T2* effects, as these are refocused in a position dependent manner. This in turn enables accurate T2 mapping from the magnitude-mode MSE SPEN data. Alternatively, the phase encodings derived from the alternating even/odd SPEN echoes due to the effects of the blipped gradients, provide an opportunity to introduce an additional z phase-encoding (PE), from which single-shot volumetric reconstruction is feasible as explained in Fig. 1C.RESULTS
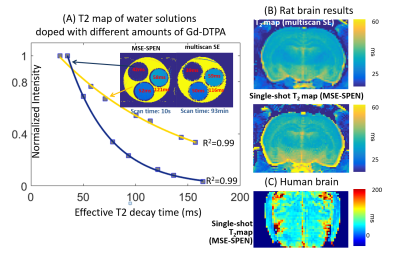
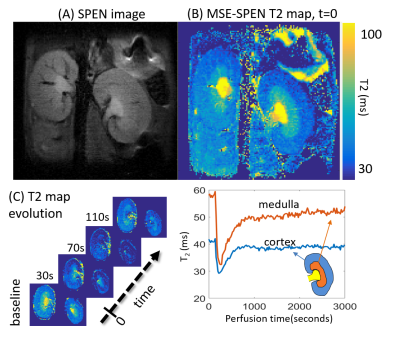
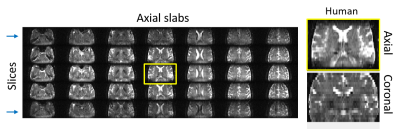
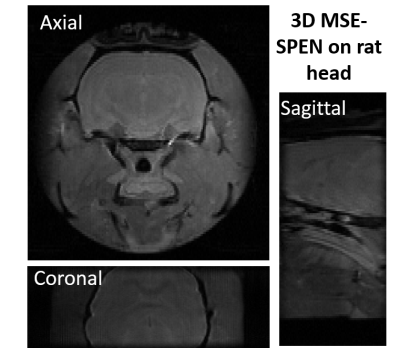
Figure 2A compares T2 values arising in a phantom containing different Gd-DTPA concentrations, when mapped by the new single-shot MSE-SPEN approach and by a conventional multi-shot spin-echo test. The good agreement shown by this phantom is also reproduced by results collected on an ex-vivo rat once acquired by multiscan SE and once by MSE-SPEN (Fig.2B); shown in Fig.2C are MSE-SPEN results acquired on a healthy human volunteer. The fast T2 mapping of the ensuing setting could be useful for functional or perfusion settings. The latter scenario was investigated by administering a contrast agent to a live anesthetized mouse, and using MSE-SPEN to generate real-time 2D maps of the animal’s T2 changes. Figure 3 shows abdominal results observed before and after the injection of GdDTPA. The animal’s kidneys evidence a strong T2 shortening during the wash-in of the contrast bolus, and subsequent T2 lengthening with its washout. These dynamics were different for different regions, with the kidney’s cortex exhibiting lower basal end-levels than its medulla. Another possibility opened by MSEs, includes accelerating the acquisition of 3D acquisitions. The number of SPEN acquisitions the MSEs could generate in this manner was 7 in the preclinical and 3 in human scanners – insufficient for digitizing a complete FOV with sufficient resolution. Figure 4 shows, however how, in combination with partial-Fourier processing, MSE-SPEN could still successfully accelerate 3D human acquisitions (despite a reliance on a ca. 20% oversampling for each slab). Figure 5 shows the results of a slightly different z-phase encoding approach applied to image a rat head. In this case the full FOVz was targeted in each scan; this, with the acquisition of 5 echoes, allowed us to collect a 3D image with 200µm isotropic resolution in sixteen shots (this time without partial FT).CONCLUSION
Single scan MSE-SPEN is a robust technique for accelerating volumetric or parametric acquisitions, capable of producing parametric 2D maps or delivering 3D volume images, in relatively short times. Minor modifications of the sequences here presented can also be conceived for encoding T2* information, for the single-shot mapping of fields, susceptibilities or chemical shifts, or for real-time diffusivity/flow measurements. CAIPI-based schemes [7] in combination with parallel receive acquisitions could also enable the execution of the multi-slab experiments here presented in a single-shot.Acknowledgements
We are grateful to Dr. Amir Seginer and Dr. Zhiyong Zhang for valuable discussions. Financial support from the Minerva Foundation (#712277), the Kimmel Institute for Magnetic Resonance and the Perlman Family Foundation (Weizmann) are gratefully acknowledged.References
[1] Tuzzi E et al. “In-vivo and ex-vivo R2* and Quantitative Susceptibility Mapping in Alzheimer’s Disease at Ultra-High Magnetic Field compared to Histology.” In Proceedings of the 25 annual meeting ISMRM 2017.
[2] Kharatishvili I et al. ”Quantitative T2 mapping as a potential marker for the initial assessment of the severity of damage after traumatic brain injury in rat.” Exp Neurol. 2009 May; 217(1):154-64.
[3] Pohlmann A et al. “High temporal resolution parametric MRI monitoring of the initial ischemia/reperfusion phase in experimental acute kidney injury. PLoS One. 2013;8(2):e57411
[4] Kumar D et al. “Gradient and Spin Echo (GRASE) as an Alternative to Multi Echo Spin Echo (MESE) acquisition for Myelin Water Fraction Imaging” In Proceedings of the 25 annual meeting ISMRM 2017.
[5] Ben-Eliezer N et al. “High-definition, single-scan 2D MRI in inhomogeneous fields using spatial encoding methods.” Magn Reson Imaging 2009;28(1):77-86.
[6] Schmidt R et al. “New spatiotemporal approaches for fully refocused, multislice ultrafast 2D MRI.” Magn Reson Med. 2014 Feb;71(2):711-22.
[7] Setsompop K et al. “Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty.” Magn Reson Med. 2012 May;67(5):1210-24.
Figures