0373
Baseline Risk Factors Suggestive of Advanced Vascular Disease Predict Differences in Brain CBF Changes After Carotid Revascularization Surgery1HMS / BIDMC, Boston, MA, United States, 2TUFTS Medical School, Boston, MA, United States, 3SUNY Binghamton, Binghamton, NY, United States, 4Stanford School of Medicine, Stanford, CA, United States, 5University of Arizona, Tucson, AZ, United States
Synopsis
Carotid stenosis is a risk factor for stroke. A number of risk factors are associated with stroke. Our work shows specific risk factors that predict differences in brain perfusion using ASL MRI technique after carotid revascularization surgery. Specifically elevated systolic blood pressure, chronic renal insufficiency, and history of prior stroke have mpacts on initial baseline to immediately post surgery prefusion increase, and baseline elevated cholesterol and body mass index show differences in 1 day to 6 months post operation. This is in contrast to hypertension, smoking and diabetes, which did not show significant predict relationships. Our work suggests that these risk factors may be more closely related to cerebrovascular dysfunction.
INTRODUCTION
Carotid stenosis is responsible for up to 20% of strokes in adults.1 Carotid artery stenting (CAS) and carotid endarterectomies (CEA) can decrease stroke risk, but have also been associated with postoperative cognitive impairment independent of cardiovascular risk factors, age, or perioperative complications.2 Some postoperative MRI-based perfusion studies have suggested that variations in CBF are associated with cognitive impairment,3 but there is a relative paucity of data evaluating longitudinal CBF changes and cognitive impairment using arterial spin labelling (ASL) MRI.4 We hypothesize that ASL patterns of CBF can be used to predict cognitive changes from carotid surgical interventions.METHODS
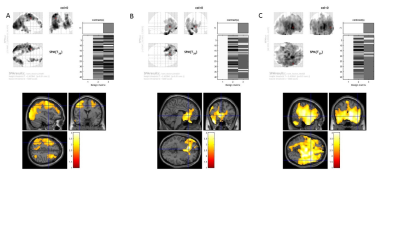
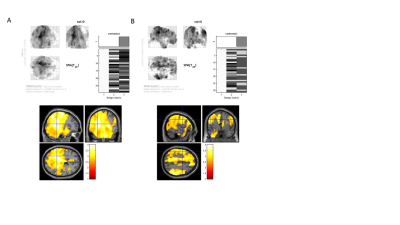
Under an IRB-approved and HIPAA-compliant protocol, 53 veterans scheduled to undergo carotid interventions (CAS or CEA) at the Veterans Affairs Palo Alto Health Care System were prospectively enrolled into the study. Indications for surgery included severe asymptomatic carotid artery stenosis (>80%) or moderate-to-severe stenosis (>60%) with focal neurological symptoms. In addition to pre-operative and post-operative imaging, subjects underwent neuropsychological testing (prior to, one month after, and 6 months after surgery) that assessed different cognitive functions [i.e., Rey's Auditory Verbal Learning Test (RAVLT),5 Delis–Kaplan Executive Function System (DKEFS),6 Controlled Oral Word Association Test (COWAT)7]. Neuroimaging followed a clinical protocol: use of a GE Discovery MR750 3.0 T MRI scanner (G.E., Waukesha, WI, USA), volumetric 3D fast spoiled gradient echo (TE Min Full; Flip angle 11 degrees; voxel size 1.2mm isotropic; TI 400; FOV 27 cm; NEX 1; Matrix 256x256), and whole brain 3D PCASL ASL imaging (axial acquisition, Freq FOV 24, Slice thickness 4-5mm, 6-8 arms, scan locs 36, NEX 3-4, BW 62.5 PLD 2525.0). CBF maps were generated from product ASL images and were then normalized using a previously reported method.8 Image subtraction analysis was performed on scans taken before, immediately after, and 6 months after surgery. These difference images were then used to compute general linearized model (GLM) contrasts using Statistical Parametric Mapping 8 software.RESULTS
All subjects demonstrated an overall increase in cerebral perfusion immediately after surgery (p<0.01). In particular, the non-surgical (i.e., contralateral) side demonstrated a larger increase in perfusion relative to the surgical side (p<0.01). All subjects demonstrated an overall decrease in perfusion from immediately after to 6 months after surgery (p<0.01). The nonsurgical (contralateral) side demonstrated a larger decline in perfusion relative to the surgical side (p<0.01). Notably, there were no significant differences in perfusion 6 months after surgery compared to before surgery (p<0.01). Preoperative elevated systolic blood pressure, presence of chronic renal insufficiency, history of prior stroke were correlated with a reduced gain in perfusion immediately after surgery. Hypercholesterolemia was correlated with a diminished reduction in perfusion from post surgery to 6 months after. Elevated BMI was associated with a greater reduction in CBF from post surgery to 6 months after.DISCUSSION
Using ASL MRI, we observed a biphasic perfusion pattern in the short-term period after carotid revascularization surgery, with an initial increase in cerebral perfusion followed by a decrease in perfusion, as has been seen in other studies using ASL, PET and SPECT. The transience and magnitude of the perfusion changes likely reflects preservation of cerebrovascular autoregulatory functions.9 Conversely, our findings that elevated systolic blood pressure, prior stroke, and chronic renal insufficiency predicted smaller amplitude perfusion changes suggesting an impairment in cerebrovascular autoregulation and vascular reserve. The fact that individual preoperative risk factors, such as hypertension, diabetes, and smoking did not predict differences in perfusion changes also suggest that more advanced derangements of vascular autoregulation may be required before significant differences in perfusion changes are observed. The fact that elevated cholesterol at baseline was associated with a lesser reduction in perfusion and baseline elevated BMI was associated with a greater reduction in perfusion from immediately post surgery to 6 months, also suggests altered vascular mechanics. More time points are needed to characterize the relation between cognition and cerebral perfusion.Conclusion
Baseline risk factors suggestive of advanced vascular impairment can be more predictive of differences in brain perfusion response as measured by ASL MRI after revascularization surgery. A better understanding of the relationship between these risk factors and cerebral vasoreactivity will enhance the prognostic utility of perfusion imaging modalities in various neurologic conditions.Acknowledgements
No acknowledgement found.References
1. Linfante I, Andreone V, Akkawi N, et al. Internal carotid artery stenting in patients over 80 years of age: single-center experience and review of the literature. J Neuroimaging 2009;19:158-163. 2. Paraskevas KI, Lazaridis C, Andrews CM, et al. Comparison of cognitive function after carotid artery stenting versus carotid endarterectomy. Eur J Vasc Endovasc Surg 2014;47:221-231. 3. Wilson DA, Mocco J, D'Ambrosio AL, et al. Post-carotid endarterectomy neurocognitive decline is associated with cerebral blood flow asymmetry on post-operative magnetic resonance perfusion brain scans. Neurol Res 2008;30:302-306. 4. Wang T, Sun D, Liu Y, et al. The impact of carotid artery stenting on cerebral perfusion, functional connectivity, and cognition in severe asymptomatic carotid stenosis. Front Neurol 2017;8:403. 5. Bean J. Rey Auditory Verbal Learning Test, Rey AVLT. In: Kreutzer JS, DeLuca J, Caplan B, eds. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York; 2011:2174-2175. 6. Homack S, Lee D, Riccio CA. Test review: Delis-Kaplan executive function system. J Clin Exp Neuropsychol 2005;27:599-609. 7. Patterson J. Controlled Oral Word Association Test. In: Kreutzer JS, DeLuca J, Caplan B, eds. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York; 2011:703-706. 8. Dai W, Alsop D. Robust, Accurate and Automated Normalization of 3D Arterial Spin Labeling Brain Images. ISMRM. Milan, Italy: ISMRM; 2014. 9. Moulakakis KG, Mylonas SN, Sfyroeras GS, et al. Hyperperfusion syndrome after carotid revascularization. J Vasc Surg 2009;49:1060-1068. 10. Reagh ZM, Roberts JM, Ly M, et al. Spatial discrimination deficits as a function of mnemonic interference in aged adults with and without memory impairment. Hippocampus. 2014;24(3):303–314. 11. Babiloni C, Vecchio F, Mirabella G, et al. Hippocampal, Amygdala, and Neocortical Synchronization of Theta Rhythms is Related to an Immediate Recall During Rey Auditory Verbal Learning Test. Human Brain Mapping 2009;30:2077–2089. 12. Newman LM, Trivedi MA, Bendlin BB, et al. The Relationship Between Gray Matter Morphometry and Neuropsychological Performance in a Large Sample of Cognitively Healthy Adults. Brain Imaging Behav. 2007;1(1-2):3–10. 13. Katzev M, Tüscher O, Hennig J, et al. Revisiting the functional specialization of left inferior frontal gyrus in phonological and semantic fluency: the crucial role of task demands and individual ability. J. Neurosci. 2013;33:7837–7845. 14. Pa J, Possin KL, Wilson SM, et al. Gray matter correlates of set-shifting among neurodegenerative disease, mild cognitive impairment, and healthy older adults. J Int Neuropsychol Soc. 2010;16(4):640–650. 15. Adolfsdottir S, Haasz J, Wehling E, et al. Salient Measures of Inhibition and Switching Are Associated With Frontal Lobe Gray Matter Volume in Healthy Middle-Aged and Older Adults. Neuropsychology 2014;28(6):859–869. 16. Sundt TM Jr, Sharbrough FW, Piepgras DG, et al. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc. 1981;56(9):533–43.Figures