0372
Sensitivity and specificity of cerebrovascular reactivity in predicting surgical decisions in Moyamoya patients1Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
Moyamoya disease (MMD) is characterized by chronic occlusion of the distal intracranial internal carotid arteries and can be treated by revascularization surgery. At present, surgical decisions are primarily based on symptomatology and imaging studies such as DSA and SPECT. However, current procedures are costly, invasive, and qualitative. Here we applied a novel iVas-MRI technique that provides quantitative assessment of multiple hemodynamic parameters in a single scan of 9 minutes, and examined its sensitivity and specificity in predicting surgical decisions. Our results showed that iVas-MRI has an overall accuracy of 0.93 in predicting surgical decisions in MMD.
Introduction
Moyamoya disease (MMD), characterized by chronic occlusion of the distal intracranial internal carotid arteries (ICAs), is a progressive unilateral or bilateral arterial stenotic disease leading to recurrent stroke1. Revascularization surgery to augment blood supply to the cerebral hemisphere in question is the standard treatment in MMD2. At present, the decision of when to proceed with surgery is primarily based upon severity of clinical symptoms and imaging modalities such as DSA and SPECT. However, the current decision-making process for each brain hemisphere is costly, invasive, and sometimes subjective. It is plausible that recent advances in MRI hemodynamic imaging methods can provide a more objective index for surgical decision-making. Recently, an integrated vascular (iVas) MRI was developed to provide quantitative mapping of cerebrovascular reactivity (CVR), cerebral blood volume (CBV), and bolus arrival time (BAT) without using contrast agent3. In this study, we applied this technique in MMD patients, and evaluated the ability of iVas parametric maps to predict surgical decisions in such patients.Methods
Patients: Fourteen MMD patients (40.4±9.6y, range 24-60y, 1 male) were scanned on 3T MRI (Philips). Each patient had at least one hemisphere pending decision regarding surgery at the time of MRI, with a total of 20 hemispheres available for testing our hypothesis (the remaining 8 hemispheres had already undergone surgery at the time of research MRI). Independent of the research MRI, neurosurgeons made surgical decisions on these hemispheres following their standard practice.
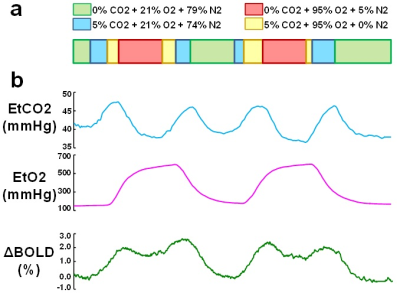
Hemodynamic mapping with iVas-MRI: During iVas-MRI, a concomitant CO2/O2 challenge was performed while BOLD images were continuously collected3. The timing of the CO2 and O2 paradigms were optimized previously3, allowing CO2 and O2 levels to be modulated independently (Figure 1). The BOLD sequence used the following parameters: TR/TE=1510/21ms, 3.2mm isotropic voxels, scan duration=9.3 min.
Data processing: Following the analysis method described previously3, BOLD images and physiological recordings of end-tidal (Et) CO2 and O2 traces were used to calculate maps of CVR (based on BOLD signal change to EtCO2 change), CBV (based on BOLD signal change to EtO2 change), and BAT (based on the time lag between physiological recordings and BOLD signal). BAT were estimated separately for CO2 and O2 gases. For each hemisphere under consideration, parametric values from the ICA perfusion territory (based on a perfusion atlas by van Laar et al.4) were spatially averaged.
Statistical analysis: The 20 hemispheres under consideration were divided into two groups based upon the surgical decisions made by the treating neurosurgeons blinded to research data. CVR, CBV and BAT values were compared between the groups using two-sample t-tests. A p<0.05 was considered significant. If a significant group difference was found for a parameter, a receiver-operating-characteristic (ROC) curve was generated and the area-under-the-ROC-curve (AUC) was calculated to evaluate the performance of the MRI index in predicting surgical decisions.
Results and Discussion
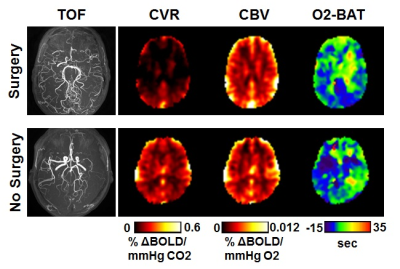
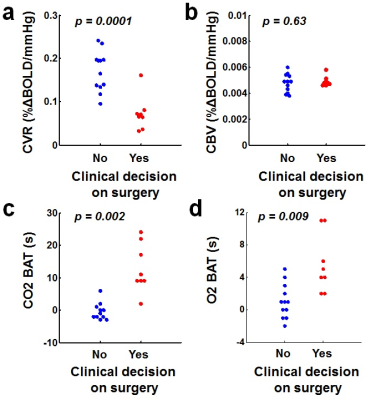
Out of the 20 hemispheres under consideration, 8 were diagnosed as surgical and 12 considered medical. Figure 2 shows iVas parametric maps from one patient who was recommended to receive revascularization surgery on both hemispheres and another patient in whom revascularization surgery was not recommended (despite clear arterial stenosis, see red arrow in Figure 2). It can be seen that the patient who received surgery revealed considerable diminution in CVR presurgically while her CBV was intact, compared to the medically-managed patient. Figure 3 summarizes data from all hemispheres. CVR values in the medical and surgical groups were 0.17±0.05 and 0.07±0.04 %ΔBOLD/mmHg CO2, respectively, p=0.0001. CBV was 0.0048±0.0007 and 0.0049±0.0004 %ΔBOLD/mmHg O2, in the two groups, respectively, p=0.63. CO2-BAT and O2-BAT were 0.17±3.13s and 1.08±2.11s for the medical group, and 12.88±7.47s and 5.63±3.58s for the surgical group, both with significant group differences (p=0.002 for CO2-BAT and 0.009 for O2-BAT).
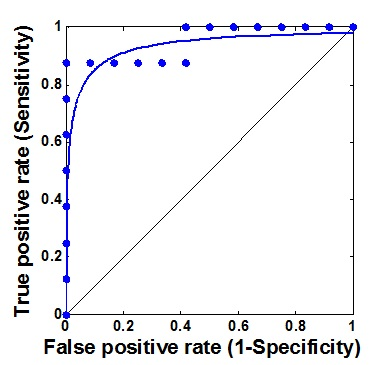
ROC analyses were performed for CVR and BATs. The ROC curve of CVR is shown in Figure 4 with an AUC of 0.93, indicating an excellent accuracy (AUC=0.9~1) of CVR as a predictor of indication for surgical treatment. When using a cutoff of 0.10 %ΔBOLD/mmHg, the sensitivity and specificity was found to be 88% and 100%. The AUC values for CO2-BAT and O2 BAT were both 0.87, which is considered a good accuracy (AUC=0.8~0.9).
Conclusion
We applied a novel iVas-MRI technique to measure CVR, CBV and BAT concomitantly in a single scan in MMD patients. It was found that brain hemispheres that required revascularization surgeries had lower CVR and longer BAT, compared to medically-managed hemispheres. ROC analyses suggested that CVR had an excellent accuracy in predicting surgical decisions. iVas-MRI may be a more cost-effective and reproducible method to select between medical and surgical treatments for MMD patients.Acknowledgements
No acknowledgement found.References
1. Chiu D, Shedden P, Bratina P, Grotta JC. Clinical features of moyamoya disease in the United States. Stroke 1998; 29: 1347-1351.
2. Macyszyn L, Attiah M, Ma TS, Ali Z, Faught R, Hossain A, Man K, Patel H, Sobota R, Zager EL, Stein SC. Direct versus indirect revascularization procedures for moyamoya disease: a comparative effectiveness study. J Neurosurg 2017; 126: 1523-1529.
3. Liu, P., Welch, B.G., Li, Y., Gu, H., King, D., Yang, Y., Pinho, M., Lu, H., 2017. Multiparametric imaging of brain hemodynamics and function using gas-inhalation MRI. Neuroimage 146, 715-723.
4. van Laar PJ, Hendrikse J, Golay X, Lu H, van Osch MJ, van der Grond J. In vivo flow territory mapping of major brain feeding arteries. Neuroimage. 2006;29(1):136-44.
Figures