0370
The effect of breath-hold on cardiovascular pulse in the brain - a multimodal MREG study.1OFNI/Radiology, Oulu University Hospital, Oulu, Finland
Synopsis
MREG enables critical sampling of human brain physiology. This study reveals novel pulsation changes during repeated breatholds of 32 seconds. Cardiorespiratory control centers in the brain stem show increased pulsation amplitude during the end of the breathold. Also the overall cardiac pulse propagation in the brain alters markedly by breathold, especially when repeated. The findigs show that the MREG data enables spatially accurate estimation of the status of the cardiovascular pulsation which is the primary driver of glymphatic brain clearance.
Introduction
Recently discovered glymphatic brain clearance is essentially driven by physiological pulsations1. Magnetic resonance encephalography (MREG) can separate physiological pulses such as cardiovascular, respiratory and vasomotor waves from human brain pulsations data2. In this study we examined the effects of breathold to cardiovascular brain pulses with multimodal MREG data in the human brain3.Materials and Methods
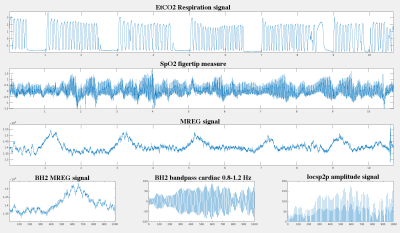
10 healthy volunteers (5 females, age 34 +/-10 years ) were imaged with 3 T Skyra MREG sequence 3 D whole brain (TR 100 ms,TE 3.6 ms, flip angle 5) during ad lib normoventilated (NV) resting state for 10 min & breath-hold 5 x 32 sec with 1.5 min between breath-holds (BH). Data was processed with typical FSL pre-peocessing steps. The MREG data was sliced into ad lib NV and BH periods with matching time lapse from the scan onset. The data was band-pass filtered based on individual cardiac (0.8 -1.2 Hz) monitoring data. Voxel level changes in cardiac pulsation ampitudes were measured from the by identifying cardiovascular pulsation amplitudes from peak to pulse thorough analysis (locsp2p). Continuous amplitude waves were done by enveloping loscp2p signal and z-scored for comparing BH changes to NV at voxel level. Brain 3D pulsation mapping was performed with QPP analysis that detects and averages repeating spatiotemporal patterns2. The BH cardiac pulsation maps were subtracted from NV-pulsation maps aligned to MNI space. The 3d+time cardiovascular pulse video correlations were analysed with fslcc in order to map dynamic changes in cardiovascular pulse spread as a function of BH.Results
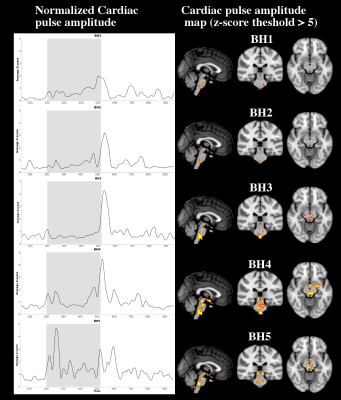
At the end of each 32 sec BH, the cardiovascular pulse amplitude increases significantly (> 2.3 std) in the brain stem areas of cardiorespiratory control (Fig.2.). In the cortex, the BH does not increase cardiovascular pulsation, even though the BOLD signal presents typical increase towards the end of each BH. Successive BH’s also tend to increase the cardiac pulsation amplitude even further. The first BH's show an increase in the respiratory centres become also start to show elevated pulsation in the thalamic and upper brain stem areas, c.f. Fig 2.
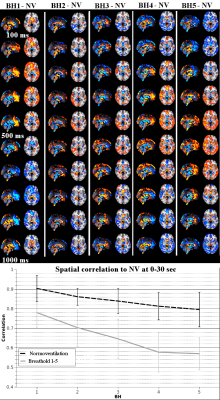
We previously described a propagating cardiac pulse wave in the brain2. Also the anatomical spread of
this cardiovascular pulse wave in
the whole brain alters as a
function of
the BH in Fig.3. The pulse effects become more stronger at the cortical areas and perivascular central areas lose their power with repeating BH numbers. Compared to original normoventilation status the 3d+time correlation falls to 0.57 which is significant compared to the drop in correlation over time detected without BH.
Conclusion
The MREG was able to detect an increase in the cardiac pulse amplitude in the cardiorespiratory control centers of the brain stem, which later extended to thalamic areas in successive breatholds. The cardiovascular pulsation amplitude was not detected anywhere else in the brain dispite normal BH-induced vasodilation detected in the BOLD signal. Furthermore, the spatial propagation of the cardiac impulse throughout the brain alters due to breathold and successive BH’s further increase this change probably in relation to CO2-build up in the tissue.Acknowledgements
JAES foundation grant is cordially acknowledged.References
1. Nedergaard M (2013) Neuroscience. Garbage truck of the brain. Science. 340(6140):1529-30.
2. Kiviniemi V, et al (2015) Ultra-fast magnetic resonance encephalography of physiological brain activity – Glymphatic pulsation mechanisms JCBFM 2016 Jun;36(6):1033-45
3. Pattinson et al., Determination of the human brainstem respiratory control network and its cortical connections in vivo using functional and structural imagin. NeuroImage 44 (2009) 295–305.
Figures