0365
Self-gated free-breathing cine DENSE imaging by adaptively reducing residual T1-echo energy1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Radiology, University of Virginia, Charlottesville, VA, United States
Synopsis
Cine DENSE is a well-established myocardial strain imaging technique that typically requires breath-holding. In this study, we developed a self-gated free-breathing adaptive acquisition algorithm that reduces free-breathing artifacts by minimizing the residual energy of the phase-cycled T1-relaxation signal. The algorithm adaptively repeats the acquisition of the k-space data with the highest residual T1-echo energy. Evaluation in 7 healthy subjects demonstrated that the method provides high quality free-breathing self-gated cine DENSE images in a clinically-reasonable scan time.
Introduction
Cine DENSE is a well-established myocardial strain imaging technique that typically requires breath-holding during image acquisition. Subtraction of phase-cycled data is utilized to suppress the artifact-generating T1-relaxation echo.1,2 With free-breathing, suppression of the T1-relaxation echo is not effective due to respiratory motion between the phase-cycled data, resulting in artifacts. We developed an adaptive acquisition that reduces artifacts by diminishing residual T1-echo energy in real-time.Methods
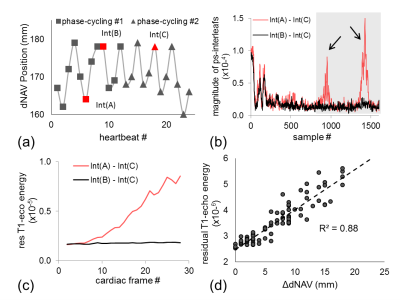
The DENSE signal includes a stimulated echo and a T1-relaxation echo, with echo centers occurring at different locations in k-space.1 Fig.1-b shows example k-space data subsequent to subtraction of phase-cycled data along a spiral trajectory. When phase-cycled data are acquired at the same position, suppression of the T1-relaxation echo is effective (black curve). However, strong residual T1-relaxation signal remains when the data are acquired at different positions (red curve). Residual energy of the T1-echo (rT1E) can be simply quantified by summing the energy over a predefined k-space range (gray area). The rT1E of free-breathing (FB) is substantially higher than that of breath-hold (BH), especially during diastole (Fig.1-c). Further, the rT1E correlates with differences in respiratory position (Fig, 1-d). We reasoned that FB artifacts can be minimized by reducing the rT1E.
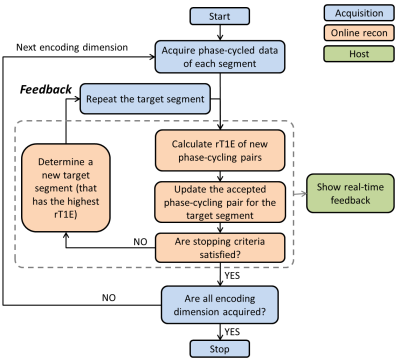
An adaptive acquisition algorithm was implemented using a 2D spiral cine DENSE sequence with a segmented spiral trajectory and localized stimulated echoes.3 As in Fig.2, the acquisition starts by acquiring a complete set of k-space data. The online reconstruction environment (ICE) calculates rT1E of each phase-cycling pair and determines whether the stopping criteria are satisfied. If so, the acquisition stops and moves to the next displacement-encoding dimension. If not, ICE determines the segment that currently has the highest rT1E (target segment). Real-time feedback is delivered to the sequence, which then repeats the target segment. When new data are acquired, they are compared to all previously acquired data of the same segment to find the best matched phase-cycling pair. Presently, the stopping criterion is an imaging time of 30 heartbeats.
Seven healthy subjects were scanned on a 3T system (Prisma, Siemens) with a 6-channel body coil and a 32-channel spine coil. FB datasets were acquired for mid-ventricular short-axis slices using: 6 interleaves per image, 2 interleaves per segment, spatial resolution of 3x3 mm2, TR = 15 ms, TE = 1.08 ms, uniform rotation of the spiral trajectory through frames, simple 3-point displacement encoding, and displacement-encoding frequency of 0.10 cyc/mm.
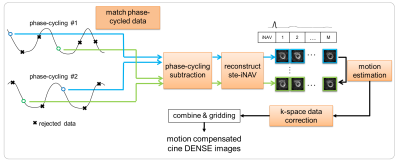
Each dataset was analyzed offline in MATLAB (Mathworks, USA), mimicking the online algorithm. Cine DENSE images were reconstructed from the identified phase-cycling pairs with minimized rT1E and subsequent motion correction, as shown in Fig.3. The quality of each reconstruction was evaluated using the signal-to-noise ratio (SNR) of magnitude images.4
Results
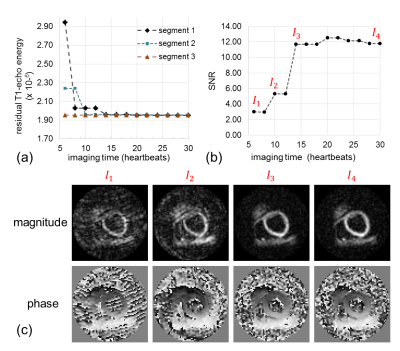
Fig.4 shows results from one volunteer demonstrating the method. The overall k-space rT1E from the best matched phase-cycling pairs decreased as the adaptive acquisition progressed and reached a low level after 15 heartbeats (a). At each time, the segment with the highest rT1E was repeated and its rT1E was reduced after the acquisition of new data. Correspondingly, the SNR of the DENSE magnitude images increased (b). Panel (c) shows images of a late diastolic frame from 4 different time points during the acquisition (I1-I4). Strong artifacts are present in I1-I2 but not in I3-I4.
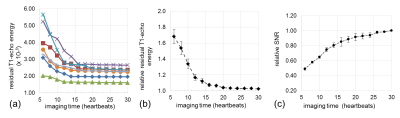
Fig. 5 summarizes the results from all subjects. The rT1E converged similarly for all subjects, although to a different value due to intrinsic k-space energy variations between subjects (a). These variations were accounted for by normalizing the rT1E to that of early systolic frames, which can be estimated at each time. The normalized rT1E converged to a similar value (1.027 ± 0.007, mean ± standard error, at an imaging time of 30 heartbeats) (b). Compared to the initial value, the relative rT1E decreased by 70% after 20-heartbeats. The relative SNR (normalized to the final reconstructed image) doubled (c).
Discussion
We developed
an adaptive algorithm for self-gated FB cine DENSE imaging. The algorithm
minimizes rT1E, which is a surrogate for motion between phase-cycled data to
reduce artifacts. In vivo experiments demonstrated that image quality increased
as rT1E decreased during the adaptive acquisition. For the specific protocol used,
the time to converge to high-quality images was 20-25 heartbeats per
displacement-encoding dimension. We found that the normalized rT1E may be a
stopping criterion to provide high image quality and shorter scan times.
Although not shown, motion estimation and compensation contributed to image
quality in a substantial number of cases. These methods are promising for
self-gated FB cine DENSE, and a clinical evaluation of the method is warranted.Acknowledgements
Research support from Siemens Healthineers, American Heart Association and the Children’s Heart Foundation.References
1. Kim D, Gilson WD, Kramer CM, Epstein FH. Myocardial tissue tracking with two-dimensional cine displacement-encoded MR imaging: development and initial evaluation. Radiology 2004;230(3):862-871.
2. Zhong X, Spottiswoode BS, Meyer CH, Kramer CM, Epstein FH. Imaging three‐dimensional myocardial mechanics using navigator‐gated volumetric spiral cine DENSE MRI. Magnetic resonance in medicine 2010;64(4):1089-1097.
3. Mills P, Chew W, Litt L, Moseley M. Localized imaging using stimulated echoes. Magnetic resonance in medicine 1987;5(4):384-389.
4. Gudbjartsson H, Patz S. The Rician distribution of noisy MRI data. Magnetic resonance in medicine 1995;34(6):910-914.
Figures