0362
Beat-by-Beat Dynamic Assessment of Myocardial Oxygenation with Highly Time-Resolved, Free-breathing, Ungated Cardiac T2 BOLD MRI Using a Low-Rank Tensor Formulation1Biomedical Imaging Research Institute, Cedars Sinai Medical Center, Los Angeles, CA, United States, 2Lawson Research Institute, London, Canada, 3Siemens Healthineers, Los Angeles, CA, United States, 4Cedars Sinai Medical Center, Los Angeles, CA, United States
Synopsis
Coronary vasodilation and the ensuing myocardial hyperemia following the administration of a provocative stressor is a dynamic process. However, established perfusion methods are confounded by contrast accumulation and lack the temporal resolution to accurately evaluate the process. BOLD CMR is an emerging method for monitoring myocardial perfusion without contrast agents, but the current methods are slow. We developed a non-ECG-gated, free breathing, beat-to-beat, cardiac/respiratory phase-resolved, T2-based BOLD CMR sequence at 3T using a low rank tensor framework to enable highly time-resolved assessment of coronary reactivity. We tested the proposed technique in an animal model with and without coronary disease.
Introduction
Coronary vasodilation and the ensuing myocardial hyperemia following the administration of a provocative stressor is a dynamic process. Noninvasive assessment of this process can provide important insights into the vasomotor activity of coronary vessels1, which is known to be impaired in numerous pathologies that affect the heart. Current methods for ascertaining myocardial perfusion rely on monitoring the accumulation and passage of exogenous contrast agents. However, slow clearance of these contrast agents limits true assessment of time-resolved changes in blood flow in response to a coronary vasodilator. A subtle consequence of this is reflected in the empirical delay set by the operator between the start of the vasodilator infusion and imaging data acquisition in first-pass perfusion (FPP) exams, which can compromise the capture of peak coronary vasodilaton in some subjects. Hence, FPP approaches effectively limit blood flow assessments to two physiological states: one at rest; and another at some presumed peak vasodilatory state. Cardiac BOLD MRI is an emerging method for probing myocardial perfusion without contrast agents2,3. However, current BOLD CMR techniques are slow; thus, they do not have the temporal resolution to report on the vasodilatory changes in the heart that occur at a much faster time scale. Moreover, they are sensitive to breathing motion and have low tolerance to rapidly varying heart rates (R-R intervals) following the administration of stress agents. To overcome these limitations and to enable rapid time-resolved assessment of myocardial perfusion, we developed a non-ECG-gated, free breathing, beat-to-beat, respiratory and cardiac phase-resolved, T2-based BOLD CMR sequence at 3T using a low rank tensor (LRT) framework4,5. Specifically, we tested whether the myocardial perfusion dynamics can be captured using the proposed method in intact animals before and after the administration of regadenoson, a commonly used coronary vasodilator. We also applied the proposed technique for the assessment of dynamical changes in regional perfusion in an animal model with coronary disease.Methods
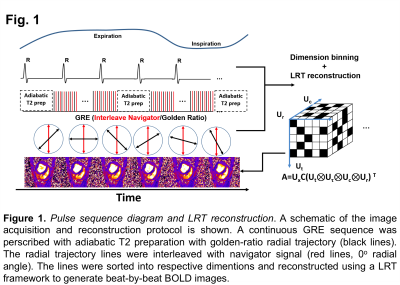
The proposed cardiac BOLD MRI approach was developed based on a LRT formalism (acquisition and reconstruction) that was previously used for ungated cardiac T1 mapping. It is composed of 3 parts: (i) adiabatic T2 preparation that is repeated at a fixed interval to ensure consistent T2 weighting; (ii) repeat acquisition of a set of central k-space lines with GRE readout every other TR to serve as respiratory and cardiac navigators for reconstruction; and (iii) interleaving of a set of golden-ratio radial GRE readout lines with the navigator lines serving as LRT training data. In brief, we modeled a high-dimensional cardiac image space (cardiac motion(Uc), respiratory motion(Ur), T1 recovery time(Ut), and time after excitation(Ut)) as a low-rank tensor that is partially separable. The complete tensors of all subspaces were recovered from the frequently sampled navigator signal using LRT completion. Subsequently cardiac and respiratory phased-resolved, beat-to-beat cardiac BOLD images were reconstructed. Anesthetized dogs (intact, n=2; and with chronic myocardial infarction, n=2) underwent continuous acquisition in a 3T MRI system (Siemens, Verio) that began 1-minute prior to regadenoson injection (2.5mg/kg; duration of injection=30 sec) and ended 6 minutes after regadenoson injection. The data acquisition and reconstruction schemes are illustrated in Figure 1. Sequence parameters were: scan time: 6 mins, delay between T2prep=800ms; TE(T2prep time)=60ms; GRE readout(TE/TR=1.4/3.3ms, flip angle=12°, FOV=270x270mm2, in-plane resolution: 1.7x1.7mm2, 1 slice of thickness: 6 mm).Results
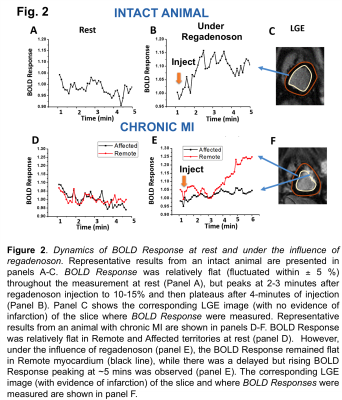
Figure 2 shows a representative time-series of normalized BOLD Response (defined as the BOLD signal normalized by the mean BOLD signal over the first 10 seconds of acquisition) at rest and during regadenoson injection from an intact animal and an animal with chronic MI. LGE images show the region where the BOLD responses were measured. In the healthy myocardium(Fig.2A, B and C) BOLD Response was relatively stable at rest but, under the influence of regadenoson, it steadily increased over a period of 2-3 minutes and plateaued to more than 10%. In animals with chronic MI (Fig.2D, E and F), rest BOLD Response was also relatively stable in the infarcted and remote myocardium. However, following regadenoson injection, there was no response in the affected(infarcted) myocardium; but in the remote myocardium, a noticeably delayed hyperemic BOLD Response was observed (compared to intact animals), which peaked ~5 minutes after regadenoson injection and plateaued to above 25%. Similar responses were observed in the other animals as well.Conclusion
The proposed BOLD approach is the first to enable noninvasive, time-resolved, interrogation of vasomotor differences in vascular beds in health and disease. We envision that this approach has the capacity to open the door for exploring novel insights into coronary circulation in health and disease.Acknowledgements
This work was supported in part by a grant from NIH (R01-HL091989)References
1. E B Ringelstein,et al., "Noninvasive assessment of CO2-induced cerebral vasomotor response in normal individuals and patients with internal carotid artery occlusions."Stroke. 1988;19:963-969
2. HJ. Yang, et al., "Towards Reliable Non-Contrast Enhanced MR-based Myocardial Perfusion Imaging: Myocardial BOLD MRI Using Late Effects of Regadenoson with Simultaneous 13N-ammonia PET Validation in a Whole-body Hybrid PET/MR System"in Proc. Int. Soc. Magn. Reson. Med., 2016
3. HJ. Yang, et al., ” Assessment of Myocardial Reactivity to Controlled Hypercapnia with Free-breathing T2-prepared Cardiac Blood Oxygen Level–Dependent MR Imaging” Radiology.2014 Apr 17:132549
4. A. G. Christodoulou et al., "3D dynamic T1 mapping of the myocardium using a time-varying subspace," in Proc. Int. Soc. Magn. Reson. Med., 2015, p. 2614.
5. A. G. Christodoulou et al., " A general low-rank tensor framework for high-dimensional cardiac imaging: Application to time-resolved T1 mapping " in Proc. Int. Soc. Magn. Reson. Med., 2016