0359
Feasibility of Ultra-high Simultaneous Multi-slice and In-plane Accelerations for Cardiac MRI Using Outer Volume Suppression and Leakage-Blocking Reconstruction1Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3Computer Assisted Clincial Medicine, University of Minnesota, Mannheim, Germany
Synopsis
Simultaneous multi-slice (SMS) imaging has gained increasing interest for enabling high scan-time acceleration at the cost of minimal loss in SNR. However, its applications in cardiac MRI have been limited, as the feasible acceleration is restricted by unfavorable coil geometry. In this study, we investigate the use of outer-volume suppression (OVS) in combination with CAIPIRINHA to promote dissimilarities among the multi-bands. We propose a time and SAR efficient multi-band scheme for OVS and apply these techniques with a leakage blocking reconstruction to increase the feasible acceleration in cardiac cine and perfusion imaging. Combining these techniques, we achieve clinical image quality with 5 fold SMS acceleration in Cine and 16-fold spatial-only acceleration in perfusion MRI.
Introduction
Simultaneous multi-slice/Multi-band (SMS/MB)1 imaging has been introduced as a means for scan-time reduction, where the only loss in SNR is caused by coil-geometries. CAIPIRINHA2 can be utilized to further reduce g-factors, using differential FOV shifts in each multi-band. However, applications in cardiac MRI have been limited due to coil geometry and presence of extra-cardiac tissue, such as chest and back fat, which leads to residual aliasing or leakage. This challenge is further exacerbated when using additional in-plane acceleration, as in most cardiac MRI scans.
In this study, we propose a fast outer-volume suppression (OVS) method to enable higher acceleration factors in cardiac imaging. We study cardiac cine and perfusion imaging, with interleaved repeated OVS, and a spatial-only (i.e. no assumptions about dynamic imaging) leakage-blocking reconstruction to enable very high acceleration rates.
Methods
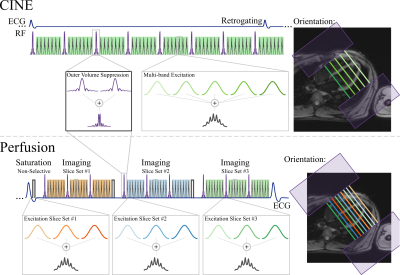
Sequence and Reconstruction: Figure 1 depicts the sequence diagrams of the proposed methods for OVS-SMS/MB imaging. In both sequences, OVS is performed using the multi-band combination of two slab-selective saturation pulses. These are used to suppress signal from parallel slabs along phase-encoding dimension. OVS is performed in an interleaved manner to ensure consistent suppression throughout acquisition. In both sequences CAIPIRINHA is performed by differentially modifying the excitation pulse phase of each respective band in a cyclic fashion. Images were reconstructed using a leakage-blocking approach, called split slice-GRAPPA (Split-SG)3.
Imaging Experiments: In-vivo imaging was performed in healthy subjects at 3T (Siemens Magnetom Prisma). First-pass perfusion imaging was performed with injection of 0.05 mmol/kg gadobutrol (Gadovist) at 4 mL/s, followed by a 10-mL saline flush; and cine imaging was performed subsequently for time-efficient scanning. Cine parameters were: TR/TE/FA=4.3/2.1ms/12°, FOV/resolution=320x320/1.7x1.7mm2, slice thickness=6mm, SMS/MB-factor=5 or 6, temporal resolution=41ms, breath-hold duration=15-17s. Fruther acceleration was assessed by retrospectively undersampling the MB=5 dataset by in-plane accelearation=2 while keeping ACS=24 reference lines. Perfusion parameters were: SMS/MB-factor=3, in-plane acceleration=4, and partial-Fourier=6/8 were utilized for an overall 16-fold acceleration. The imaging parameters were: FOV=360x360mm2, resolution=1.7x1.7mm2, slice-thickness=8mm, TR/TE/FA=2.9/1.7ms/12°, temporal resolution=110ms, saturation time=150ms.
Both sequences share the same OVS module with saturation slab=150mm (each side), 3.8ms assymmetric sinc, BWT=8, RF peak shift4 = 15%. MB-OVS preparation was interleaved between every 9 imaging pulses for perfusion and 8 for cine.
Results
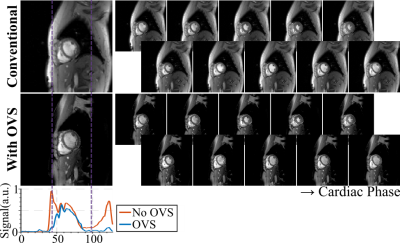
The effects of OVS are depicted in Figure 2, which shows cine images without acceleration. Thorough saturation of the periphery along the phase-encoding direction is observed in all cardiac phases. Evaluation of signal intensity along phase-encoding direction shows a profound drop outside the region-of-interest, especially in the chest and back.
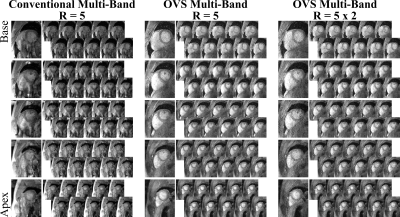
Figure 3 shows cine imaging with with SMS/MB-factor=5. Without OVS (left), substantial signal leakage onto the heart prevents diagnostic information. Excellent image quality is obtained with OVS and Split-SG reconstruction (middle), resulting in artifact-free depiction of cardiac structures through all cardiac phases and slices. With the retrospective in-plane acceleration, for total 10-fold acceleration (right), proposed approach results in leakage-free images, although increased noise amplification is observed.
Figure 4 depicts SMS/MB-factor=6 cine images. Heavy leakage artifacts cover the heart without OVS (left). Proposed approach successfully eliminates leakage artifacts (right), depicting clear myocardium blood-interface. Residual contrast variation is observed, which might hamper image evaluation.
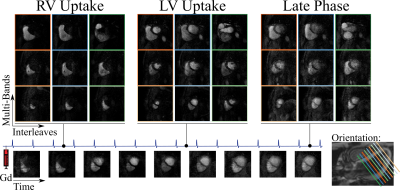
Perfusion images acquired with 16-fold spatial-only acceleration using the proposed approach are shown in Figure 5, with accurate representation of contrast dynamics across 9 slices per heart-beat. Despite low base-line SNR in the saturation-recovery sequence, no leakage artifacts are observed.
Discussion
We used OVS to reduce leakage from extra-cardiac tissues in cardiac SMS imaging. Compared to previous OVS pulses for cardiac MRI5-6, our scheme benefits from low SAR, especially important at 3T. This facilitates repeated application, ensuring homogeneous signal suppression throughout cardiac cycle. Furthermore, the brevity of the pulse minimizes disruption of the steady-state signal during the FLASH acquisition, mitigating potential artifacts that might otherwise occur.
As opposed to other methods for scan-time acceleration that are commonly used in cine MRI7 or perfusion8, proposed acquisition and reconstruction do not employ any interrelation in temporal dimension, precluding temporal blurring artifacts. Furthermore, no regularization is applied during the Split-SG reconstruction, circumventing risk of over-regularization, which may lead to patchy or blurry images.
Conclusion
The proposed MB-OVS technique with leakage-blocking reconstruction enables ultra-high scan time acceleration in cardiac MRI, without the need for regularization or exploiting temporal dependencies. MB-factors up to 5, and spatial only net-acceleration rates of 10-fold and 16-fold were achieved in cine and perfusion imaging, respectively, while maintaining diagnostic image quality.Acknowledgements
Funding: Grant support: NIH R00HL111410, NIH P41EB015894 and NSF CCF-1651825.References
1. Larkman DJ, Hajnal JV, Herlihy AH, Coutts GA, Young IR, EhnholmG. Use of multicoil arrays for separation of signal from multiple slicessimultaneously excited. J Magn Reson Imaging 2001;13:313–317.
2. Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med. 2005 Mar;53(3):684-91.
3. Cauley SF, Polimeni JR, Bhat H, Wald LL, Setsompop K. Interslice leakage artifact reduction technique for simultaneous multislice acquisitions. Magn Reson Med. 2014 Jul;72(1):93-102.
4. Auerbach EJ, Xu J, Yacoub E, Moeller S, Uğurbil K. Multiband accelerated spin-echo echo planar imaging with reduced peak RF power using time-shifted RF pulses. Magn Reson Med. 2013 May;69(5):1261-7.
5. Smith TB, Nayak KS. Reduced field of view MRI with rapid, B1-robust outer volume suppression. Magn Reson Med. 2012 May;67(5):1316-23.
6. Yang Y, Zhao L, Chen X, Shaw PW, Gonzalez JA, Epstein FH, Meyer CH, Kramer CM, Salerno M. Reduced field of view single-shot spiral perfusion imaging. Magn Reson Med. 2017. doi: 10.1002/mrm.26664.
7. Feng L, Srichai MB, Lim RP, Harrison A, King W, Adluru G, Dibella EV, Sodickson DK, Otazo R, Kim D. Highly accelerated real-time cardiac cine MRI using k-t SPARSE-SENSE. Magn Reson Med. 2013;70(1):64-74.
8. Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med. 2010;64(3):767-76.
Figures