0332
Solid-State MRI as a noninvasive alternative to computed tomography for craniofacial imaging1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Plastic Surgery, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Computed tomography (CT) imaging is the imaging modality of choice for 3D visualization of bone. However, there is growing concern about repeated exposure to ionizing radiation, in particular during infancy, for instance, in patients with craniosynostosis pre- and post-surgery. Here, we developed a dual-RF, dual-echo, 3D UTE sequence using view-sharing to minimize scan time. Images are reconstructed by combining long- and short-RF, first and second echoes, yielding soft-tissue suppressed skull images at 1.1 mm isotropic resolution in 6 minutes scan time in a human skull ex vivo and test subjects in vivo. 3D renderings display the relevant craniofacial skeleton similar to CT.
Introduction
Computed tomography (CT) enables 3D visualization of cortical bone structures with high spatial resolution, and thus has been the gold standard method for evaluation and diagnosis of craniofacial skeletal pathologies. However, ionizing radiation, and in particular, repeated scanning with this modality in pre- and post-surgery, is of concern when applied to infants and young children. As an alternative, Eley et al1 proposed a ‘black-bone’ MRI method in which low flip-angle 3D GRE imaging yields proton-density weighted contrast, thereby facilitating discrimination between bone and soft-tissue. However, in this approach bone appears with near background intensity (i.e. ‘black’) due to very short T2 and relatively low proton density, making it challenging to distinguish between bone and air. Solid-state MRI methods via ultrashort-echo-time (UTE)2 or zero-TE (ZTE)3, capable of imaging spins with very short T2, are thus promising alternatives. Wiesinger et al4 demonstrated a feasibility of a low flip-angle, short TR ZTE sequence in identifying skull bone structures via histogram-based post-processing. As an another approach to bone-specific imaging, a dual-RF based UTE method, which exploits sensitivity of bone signals to variable RF pulse widths, has recently been introduced5. Here, we developed a dual-RF, dual-echo 3D UTE sequence and accelerated imaging time via view-sharing. Its feasibility in producing accurate 3D renderings of the human skull was investigated in comparison to CT.Methods
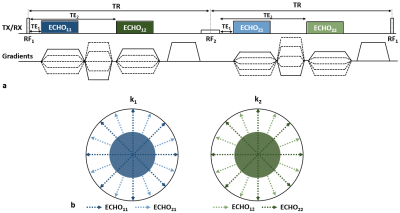
Imaging technique: Figure 1a shows a diagram of the dual-RF, dual-echo 3D UTE pulse sequence, wherein two RF pulses (RF1, RF2) differing in duration and amplitude (but equal nominal nutation angle) are alternately applied in successive TR periods along the pulse train while within each TR two echoes are acquired at short and long TEs (TE1, TE2), respectively, from the beginning of gradient ramp-up. Thus, four echoes are obtained: ECHO11, ECHO12, ECHO21, and ECHO22. Here, the subscripts represent RF and TE indices in this order (Fig. 1a). Bone proton magnetization (pertaining to bound water), due to its very short T2, exhibits a substantial level of signal decay during the relatively long duration of RF2, while soft-tissue retains nearly the same level of signal intensities over all echoes. Thus, subtraction of ECHO22 from ECHO11, when compared to the difference between ECHO11 and ECHO12, further enhances bone contrast5. In distinction to the approach in ref. 5, in the proposed method two additional signals, ECHO12 and ECHO21, are collected while radial view angles are varied every TR (instead of every two TRs as in ref. 5), leading to a two-fold increase in imaging efficiency via view-sharing. Echoes at the same TEs are combined to produce two k-space sets (k1, k2), in which central regions are composed only of ECHO11 and ECHO22 views to retain the highest and lowest bone signals, respectively, thereby maximizing bone signal specificity upon subtraction.
Data acquisition/processing: Data were acquired in a human skull ex vivo and two subjects in vivo at 3T (Siemens Prisma) using the proposed dual-RF, dual-echo 3D UTE sequence. Imaging parameters: TR/TE1/TE2 = 7/0.06/2.46 ms, RF1/RF2 durations = 40/520 μs, flip-angle = 12°, matrix size = 2563, field-of-view = 2803 mm3, voxel size = 1.1 mm isotropic, number of radial spokes = 25,000, and scan time = 6 min. Additionally, a calibration scan was performed using the method in ref. 6 to determine gradient timing delays and subsequent correction for k-space trajectory errors. Images for k1 (I1) and k2 (I2) were reconstructed using a conventional gridding algorithm. Bone images (Ibone) with minimal soft-tissue contamination were then obtained as Ibone=(I1-I2)/(I1+I2). Given the three sets of images (I1, I2, Ibone), segmentation of bone voxels was performed using ITK-SNAP7 in a semi-automatic fashion, leading to 3D renderings of the skull. For comparison, a CT scan was also performed in the human cadaveric skull with 1 mm isotropic resolution.
Results and Discussion
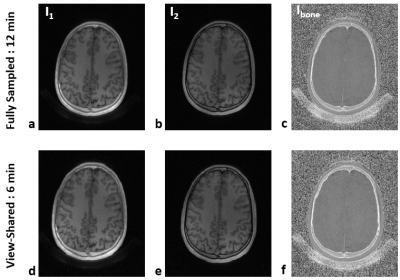
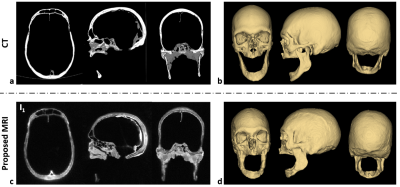
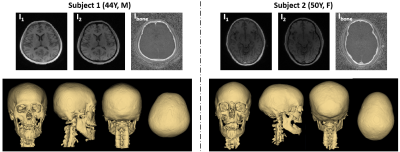
Figure 2 shows the effectiveness of the proposed view-sharing approach. Compared with full sampling5, the view-sharing scheme exhibits no appreciable loss in image quality. Figure 3 compares CT with the proposed MRI method on cadaveric human skull images, along with corresponding 3D renderings. Compared to CT, the 3D rendered images obtained with the speed-enhanced technique maintain most features over the entire head (e.g., zygomatic arch), except for appearance of some artifacts in the mandibular region. Figure 4 displays in vivo head images in two subjects. In Ibone images, bone voxels as well as inner table of the cranium are clearly visualized, and cranial and spinal bone structures are well depicted in the 3D renderings. Still, some voxels erroneously included or excluded in the renderings will require further improvement in post-processing.Conclusions
The proposed MRI-based skull imaging method, along with optimized post-processing, shows promise as a non-invasive alternative to CT for visualization of craniofacial architecture.Acknowledgements
NIH grant R01AR050068References
1. Eley KA, Mcintyre AG, Watt-Smith SR, Golding SJ. “Black bone” MRI: a partial flip angle technique for radiation reduction in craniofacial imaging. Br J Radiol. 2012;85(1011):272-278.
2. Robson MD, Gatehouse PD, Bydder M, Bydder GM. Magnetic resonance: An introduction to ultrashort TE (UTE) imaging. J Comput Assist Tomogr. 2003;27:825-846.
3. Grodzki DM, Jakob PM, Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med. 2012;67(2):510-518.
4. Wiesinger F, Sacolick LI, Menini A, Kaushik SS, Ahn S, Veit-Haibach P, Delso G, Shanbhag DD. Zero TE MR bone imaging in the head. Magn Reson Med. 2016;75(1):107-114.
5. Johnson EM, Vyas U, Ghanouni P, Pauly KB, Pauly JM. Improved cortical bone specificity in UTE MR imaging. Magn Reson Med. 2017;77:684-695.
6. Herrman K-H, Kramer M, Reichenbach JR. Time efficient 3D radial UTE sampling with fully automatic delay compensation on a clinical 3T MR scanner. PLoS ONE. 2016;11(3):e0140371.
7. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116-1128.
Figures