0330
Imaging human motor unit activity using MRI.1Institute of Genetic Medicine, Newcastle University, Newcastle upon Tyne, United Kingdom, 2Newcastle University, Newcastle upon Tyne, United Kingdom, 3Institute of Neuroscience, Newcastle University, Newcastle upon Tyne, United Kingdom, 4Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, United Kingdom
Synopsis
Neuromuscular diseases can lead to characteristic changes in the anatomy of motor units (MU). In this study we developed an MR approach to image motor unit activity. In-scanner electrical stimulation of MU was synchronized to a pulsed-gradient spin-echo imaging sequence sensitive to microscopic contraction of muscle fibers. Experimental data was compared to a simple theoretical model and scan parameters evaluated. The spatial pattern of muscle fibers forming different MU was imaged for the first time in healthy controls.
Introduction
Skeletal muscle is arranged into motor units (MU) which comprise between 5 and 1000 muscle fibers innervated by a motor nerve. Coordinated firing of MU drives mechanical contraction. Neuromuscular diseases produce characteristic changes in the anatomy of these units. Disease affecting the motor nerve (e.g. amyotrophic lateral sclerosis, ALS) leads to fewer, larger MU, whereas diseases of the muscle itself (e.g. muscular dystrophy) leads to the same number, but smaller units. Distinguishing between these is critical for clinical investigations and diagnosis.
Clinical neurophysiological assessment uses needle electrodes inserted into muscle (electromyography) or stimulation of muscle using electrical pulses (motor unit number estimation, MUNE). Both are painful, have low diagnostic yield and require patient co-operation making them difficult to perform in children or the elderly.
Previous reports identified signal voids on
diffusion imaging which were described as spontaneous focal muscle contraction
(SMAMs)1, but more correctly are muscle fasciculations driven by MU
action. Sparse, spontaneous events are extremely
challenging to study in a systematic way.
In this work we develop and validate a method to image motor unit
activity using diffusion sensitization and in-bore electrical muscle
stimulation.
Methods
A programmable stimulator (Digitimer DS5) was adapted for in-bore scanning using low-pass filters (Minicircuits) at the Faraday cage and MR compatible electrodes. The device was interfaced to the scanner for synchronization of acquisition and stimulation. A pair of 15×20 mm electrodes were placed 2 cm apart at the fibular head for stimulation of the left common peroneal nerve to the tibialis anterior (TA) using a 0.3 mS mono-polar pulse. The subject’s leg was positioned in a custom holder including a foot rest to which the foot was immobilized, in a 3T Achieva X scanner (Philips Medical Systems) and with a pair of 10cm elliptical flexible surface coils (FlexM) positioned to each side of the leg.
Scans were collected in 6 healthy volunteers using an axially oriented diffusion weighted SE-EPI sequence with sensitization in the through slice direction, along the main muscle fiber axis (1.5×1.5mm resolution, 10mm slice, TR/TE=1000/36ms, δ = 7 ms). A diffusion weighted scan was first collected shifting the acquisition time relative to the stimulus at various b-values (0 – 100s∙mm-2) to map the peak signal response in two subjects. Scan series of 60 images were then collected at peak response time with b-values of 0 (control), 10 and 20 s∙mm-2 and with current incrementing in 0.04 mA per acquisition. As current increases threshold for successive MU is reached causing new MU to fire and expanding the area of muscle involvement. Scans were repeated twice for reproducibility.
Full safety evaluation was conducted prior to human studies. The study obtained local University ethical approval and all subjects provided written consent.
Regions of interest and pixelwise analysis was performed to assess the signal response as a function of b value and the effects of incrementing current on muscle involvement.
Results
Figure 1 shows an example time course of signal change relative to the stimulus for different b values, together with the image at peak signal change (b=50s∙mm-2). Significant signal attenuation is seen associated with contraction of the muscle fibers during the diffusion sensitive period of the scan (Δ). Differences in attenuation are seen between b-values of 1 and 20 s∙mm-2 but which plateau below 50 s∙mm-2 (Figures 1 and 2). Figure 2 plots experimental signal attenuation against theory (solid line) assuming a 2% (200 μm) linear contraction of the muscle fibers.
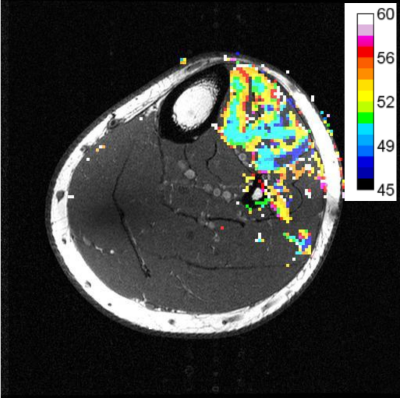
Figure 3 illustrates recruitment of motor units with stimulus current. As each unit reaches threshold current for activation the area of the signal changes expands and total signal within the whole muscle falls. This is the MRI equivalent of MUNE in electromyography.
Figure 4 illustrates an example of the spatial pattern of the MU recruitment with the color coding indicating the image volume at which that pixel fell below 3 standard deviations of the pre-stimulus signal.
Discussion
This study has demonstrated that diffusion imaging can be used to measure motor unit activity. Data is consistent with the signal mechanism being a simple contraction of the muscle during the diffusion sensitive period of the scan and shows that b-values above 50s∙mm-2 do not provide additional contrast. This method allows the distribution of motor units to be visualized in a way which is currently not possible by any other means.
Conclusion
Using in-bore electrical stimulation, these experiments demonstrate all the characteristics expected of motor unit activity and help to validate diffusion MRI as a method to non-invasively assess motor unit activity.
Acknowledgements
This work was funded by the Medical Research Council Confidence in Concept Funding Newcastle Award.References
1. Steidle G, Schick F. Addressing spontaneous signal voids in repetitive single-shot DWI of musculature: spatial and temporal patterns in the calves of healthy volunteers and consideration of unintended muscle activities as underlying mechanism. NMR in Biomedicine, 2015; 28: 801-810.Figures
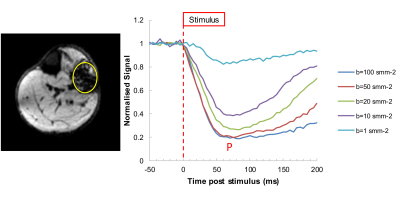
Figure 1: An example of the time course of signal change relative to the stimulus at different diffusion b-values (right) and an example image at time of peak signal (P) change (left). The sequence shows significant loss of signal associated with contraction of the muscle fibers during the diffusion sensitive period of the scan. Signal attenuation as a function of b-values plateaus beyond b=50 s∙mm-2.
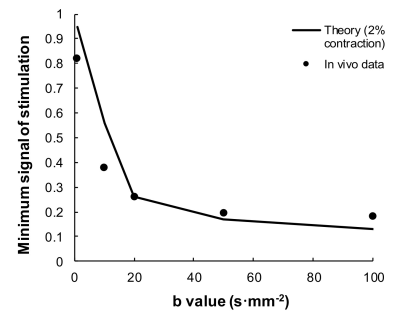
Figure 2: Peak signal attenuation derived from the data in figure 1 (dots). The solid line plots the theoretical attenuation associated with a linear contraction of muscle fibers of 2% of the slice thickness during the diffusion sensitive duration.
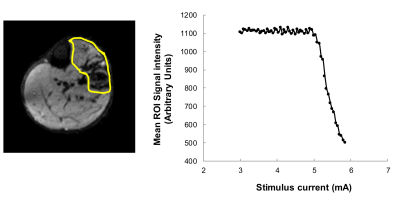
Figure 3: Example data from a diffusion weighted scan series collected with increasing muscle stimulation current. Mean image intensity across the whole stimulated muscle cross-section (left image, yellow outline) is plotted against the stimulus current (right). As each motor unit reaches threshold for stimulation the spatial signal falls another step.