0327
Intramuscular variability and sex difference in diffusion properties and 3D architecture of human lower leg muscles assessed with ultra-high-field diffusion tensor imaging and tractography1Aix-Marseille Univ, CNRS, CRMBM, Marseille, France, 2Siemens Healthcare, Erlangen, Germany, 3APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 4Institut NeuroMyoGène, Université Claude Bernard Lyon 1, INSERM, CNRS, Villeurbanne, France
Synopsis
Skeletal muscle function impairment can be associated to changes in both muscle volume and structural arrangement of fascicles/fibers within the muscle thereby leading to the reduction of muscle force production. Diffusion properties and 3D structural organization of muscle fibers were quantified from high-resolution diffusion tensor images recorded at ultra-high-field MRI (UHF-7T). These parameters were assessed regarding their intramuscular variability and sensitivity to sex difference. This application of UHF-7T might be of high interest for the assessment of muscular injuries in both athletes and patients with muscular disorders.
Introduction
Neuromuscular disorders and/or muscle injuries result in an impaired muscle function which can be due, at least partly, to changes in both muscle volume and structural arrangement of fascicles/fibers within the muscle1 leading to a reduced skeletal muscle force production.2 Quantification of both volume and architecture of the whole lower leg muscles in a single experimental session remains challenging. In the present study, we aimed at determining skeletal muscle diffusion properties and 3D structural organization using diffusion tensor imaging (DTI) at ultra-high-field (UHF) MRI (7T) and tractography of muscle fibers. Both indices were analyzed with respect to their intramuscular variability and sensitivity to sex difference using a robust registration methodology recently used to localize muscle damage.3Methods
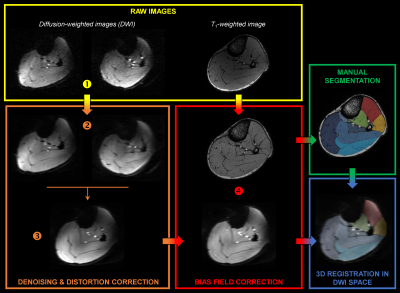
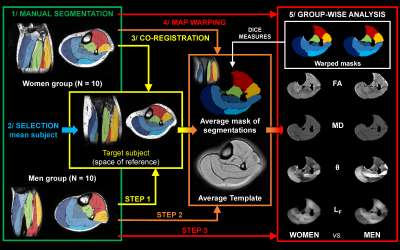
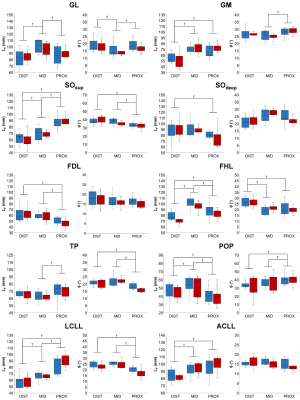
Diffusion properties and 3D architecture were characterized for the whole lower leg muscles in young healthy men (n = 10) and women (n = 10). Investigations were performed on the right lower leg using a 7T whole-body research scanner (Siemens Healthcare, Erlangen, Germany) and a 28-channel proton knee coil. Muscle volume was quantified from T1-weighted images (120 slices, FOV = 180×180 mm², matrix = 384×384, acquisition time = 8 min 34 s). Axial multidirectional diffusion weighted images were acquired with a prototype sequence employing a monopolar Stejskal-Tanner diffusion encoding scheme. Data were acquired in two opposite phase-encoding polarities to correct for distortions with the following parameters: 120 slices, FOV = 180×180 mm², matrix = 120×120, slice thickness = 1.5 mm, 30 directions, b-values = 0 and 500 s/mm², acquisition time = 7 min 54 s. Regions of interest were manually drawn for each slice over five on T1-weighted images. Interstitial slices were automatically interpolated on the basis of iterative registration tasks.4 Diffusion-weighted images were processed as described in Figure 1. Diffusion tensors were fitted on a voxel-basis in order to generate fractional anisotropy (FA) and mean diffusivity (MD) maps. Tracking parameters were adapted from previously reported criteria5 applied to initiate/stop muscle fiber tractography. The algorithm generated 2,000,000 fiber tracts with length ranging from 5 to 200 mm. From muscle fiber tractography, muscle fiber length (LF) and pennation angle (θ) were characterized. θ was determined as the angle between the muscle’s principal axis and the global direction of each fiber tracked within the muscle. Additional analyses were performed using a 3D spatial normalization (Figure 2). DICE similarity coefficients (DSCs) were used to estimate the quality of image coregistration. Each muscle volume was then divided into three equal parts (i.e., distal, central and proximal) to discriminate potential change in diffusion properties and 3D architecture parameters along muscle and across sex.Results
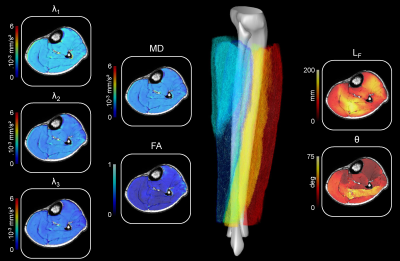
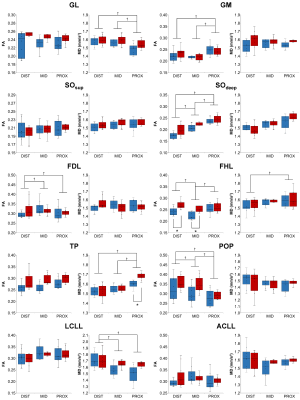
A representative example of diffusion-derived parameter maps, tractography result and 3D architecture maps is displayed in Figure 3. The DSCs associated to the coregistration of images, segmentation masks and quantitative maps were very high for all the lower leg muscles (0.91 ± 0.04). Main sex differences were found for FA in the proximal part of ACLL, SOsup and the distal part of LCLL (Figure 4). Differences independently of sex were found for several muscles – i.e., a higher FA in the proximal part for the GM and SOdeep as compared to the central and distal parts. MD was also higher in the proximal part for TP whereas it was higher in the distal part for LCLL and GL (Figure 4). LF was significantly higher in the central part for GL, FHL and POP but lower in the distal part for GM, SOsup, FHL, LCLL and ACLL. θ was lower in proximal part of SOsup, TP and ACLL (Figure 5).Discussion
Our study demonstrated that UHF MRI can be used to assess diffusion properties and 3D architecture of whole lower leg muscles from high-resolution DTI with a large volume coverage and even for deep muscles. Our results illustrated slight sex differences in muscle tissue FA, LF and θ of young subjects. Using 3D spatial normalization of images, we disclosed intramuscular differences in diffusion properties and architecture along muscles and so regardless of sex. Our results did not support previous results obtained at lower MR field on several slices6 or muscle architecture over a restricted field of view using ultrasonography.7 On that basis, one can suggest that assessment of diffusion properties and architecture should be conducted preferentially on a large volume of interest with the use of a 3D spatial normalization of images in clinical studies.Conclusion
High-resolution DTI obtained at UHF allows an accurate quantification of the structural organization of skeletal muscle fibers. This application of UHF MRI might be of high interest for the assessment of muscular injuries in both athletes and patients with muscular disorders.Acknowledgements
The authors thank the Assistance Publique des Hôpitaux de Marseille (APHM), the Centre National de la Recherche Scientifique (CNRS UMR 7339) and all the subjects who participated in the present study. This study was supported by the French IA Equipex 7T-AMI ANR-11-EQPX-0001, A*MIDEX-EI-13-07-130115-08.38-7TAMISTART, A*MIDEX ANR-11-IDEX-0001-02.References
1. Narici M, Cerretelli P. Changes in human muscle architecture in disuse-atrophy evaluated by ultrasound imaging. J Gravit Physiol. 1998;5:P73-74.
2. Lieber RL, Friden J. Clinical significance of skeletal muscle architecture. Clin Orthop Relat Res. 2001:140-151.
3. Fouré A, Le Troter A, Guye M et al. Localization and quantification of intramuscular damage using statistical parametric mapping and skeletal muscle parcellation. Sci Rep. 2015;5:18580.
4. Ogier A, Sdika M, Fouré A et al. Individual muscle segmentation in MR Images: a 3D propagation through 2D non-linear registration approaches. 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC’17). 2017:317-320.
5. Heemskerk AM, Sinha TK, Wilson KJ et al. Quantitative assessment of DTI-based muscle fiber tracking and optimal tracking parameters. Magn Reson Med. 2009;61:467-472.
6. Galban CJ, Maderwald S, Uffmann K et al. A diffusion tensor imaging analysis of gender differences in water diffusivity within human skeletal muscle. NMR Biomed. 2005;18:489-498.
7. Chow RS, Medri MK, Martin DC et al. Sonographic studies of human soleus and gastrocnemius muscle architecture: gender variability. Eur J Appl Physiol. 2000;82:236-244.
Figures