0324
Repeatability of measuring pulsatile brain tissue motion and volumetric strain with retrospectively-gated DENSE at 7T1Radiology, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
Cardiac-induced brain tissue volumetric strain (an important metric reflecting microvascular blood volume pulsation) exhibits large inter-subject variability. We measured the intra-subject repeatability of brain tissue volumetric strain over the cardiac cycle in healthy subjects at 7T using a high resolution retrospectively-gated DENSE and found that the strain curve shape and peak value were very consistent between measurements. Furthermore, we validated the peak brain tissue volume change against measured spinal CSF stroke volume at C2-C3, and observed a strong correlation. The consistent findings strengthens the potential of volumetric strain as biomarker for microvascular function in the ageing brain.
Introduction
Cardiac-induced brain tissue displacement may provide valuable information on both the microvasculature and the viscoelastic properties of the brain tissue (intrinsic activation elastography)1. Measurements of this phenomenon were successfully made with displacement encoding using stimulated echoes (DENSE)2,3. However, the derived volumetric strain curves showed a relatively wide inter-subject variability, and lacked intra-subject validation. In this study, the intra-subject repeatability of brain tissue volumetric strain in healthy volunteers was assessed and compared to spinal CSF stroke volume (sCSF) measurements as validation, as sCSF reflects the intracranial blood volume change over the cardiac cycle (CC) given the incompressibility of the intracranial volume4,5.Methods
Measurement
8 healthy subjects (3 females, mean age: 26.8±6) provided informed consent to participate in the study which was approved by the Ethical Review Board of our institution. 4D retrospectively-gated DENSE was implemented on a 7T scanner (Philips Healthcare) with a 32-channel head coil (Nova Medical), and used to measure the cardiac-induced brain tissue displacement in the RL, AP and FH directions. Cardiac gating was done with a pulse oximeter on the index finger. Subjects were shortly taken out of the scanner and then re-scanned with the same DENSE protocol. CSF flow into the spine was measured using a 2D cine-PCMRI acquisition located at the C2-C3 level. Additionally, a 3D T1-weighted FFE acquisition was made for registration and segmentation of brain tissue. See Table 1 for all scan parameters.
Analysis
Raw phase images were converted to displacement maps as previously described3. Displacement maps were registered to the T1-weighted image space using Elastix6,7. CSF, white and grey matter probability masks were created using the Computational Anatomy Toolbox (Jena University Hospital) for SPM12.
CSF, noise and artifact regions were removed from all displacement maps before computing the volumetric strain as follows. The difference between the displacement maps at the beginning and end of the CC was computed for all encoding directions. Any voxel whose absolute displacement exceeded one standard deviation of this difference map was removed. Furthermore, any voxel outside the tissue mask (having a probability less than 0.95 in the sum of the grey and white matter masks, or a non-zero value in the CSF mask) was also removed.
Volumetric strain was computed as the divergence of the masked displacement field for each cardiac phase (see Figure 1 for example images), and the mean value within the brain tissue for each cardiac phase was found. Repeatability was assessed using a Bland-Altman plot of the peak tissue volume change (pTVC) of the two measurements (calculated as the product of peak volumetric strain and tissue volume within the tissue mask). The sCSF was assessed by integrating the CSF flow over the CC8, and compared to the mean pTVC of the two measurements with a correlation plot.
Results and Discussion
Volumetric strain and CSF flow measurements were successfully attained for all subjects. The general shape of the volumetric strain curves was similar for all measurements and subjects (Figure 2). The mean absolute difference between repeated measurements was 0.14mL (see Bland-Altman plot, Figure 3). pTVC and sCSF are shown in Table 2. A strong correlation was found between sCSF and pTVC.
The results of the Bland-Altman analysis suggests good agreement between repeated measurements. The similarity between the tissue volume change (equivalently, volumetric strain) curve shapes observed amongst all subjects reflects a consistent inter-subject pattern of cardiac induced brain tissue volume change. The inter-subject peak volume change is noticeably variable. Nevertheless, the repeatability of this finding and the correlation to sCSF shows that this variation probably reflects physiological variation between subjects rather than measurement errors. This strengthens the case for brain tissue displacement as a possible biomarker for microvasculature blood volume changes, thereby providing an important window to the microvasculature which is notoriously challenging to image directly in-vivo. Thus, this metric holds potential as a means for studying blood volume pulsations in the elderly population (since arterial properties change with age), or in diseases such as cerebral small vessel disease. Calculation of volumetric strain was hampered by image artifacts3, particularly in the FH acquisitions, which currently limits regional analysis of brain tissue volumetric strain. Further improvements to this technique are necessary to remove these artifacts and boost SNR, which would allow regional strain analyses.
Conclusion
We demonstrated that cardiac induced brain tissue pulsatility has a low intra-subject variability and consistent inter-subject shapes in healthy subjects. Furthermore, it is strongly correlated to spinal CSF stroke volume. The consistency between measurements strengthens the reliability of brain tissue displacement as a potential biomarker for investigating the ageing brain in normal or diseased states.Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement n°337333.References
- Weaver JB, Pattison AJ, McGarry MD, Perreard IM, Swienckowski JG, Eskey CJ, Lollis SS, Paulsen KD. Brain mechanical property measurement using MRE with intrinsic activation. Phys. Med. Biol. 2012;57:7275–7287. doi: 10.1088/0031-9155/57/22/7275.
- Soellinger M, Rutz AK, Kozerke S, Boesiger P. 3D cine displacement-encoded MRI of pulsatile brain motion. Magn. Reson. Med. 2009;61:153–162. doi: 10.1002/mrm.21802.
- Adams AL, Luijten PR, Zwanenburg JJM. Measurements of cardiac related pulsatile volumetric strain in grey and white matter brain tissue with high resolution DENSE at 7T. In: Proceedings of the 25th Annual Meeting of ISMRM. Honolulu, USA; 2017.
- Greitz D, Wirestam R, Franck A, Nordell B, Thomsen C, Stahlberg F. Pulsatile brain movement and associated hydrodynamics studied by magnetic resonance phase imaging. Neuroradiology 1992;34:370–380. doi: 10.1007/BF00596493.
- Enzmann DR, Pelc NJ. Cerebrospinal fluid flow measured by phase-contrast cine MR. AJNR. Am. J. Neuroradiol. 1993;14:1301–1307.
- Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW. Elastix: A toolbox for intensity-based medical image registration. IEEE Trans. Med. Imaging 2010;29:196–205. doi: 10.1109/TMI.2009.2035616.
- Shamonin DP, Bron EE, Lelieveldt BPF, Smits M, Klein S, Staring M. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer’s disease. Front. Neuroinform. 2013;7:50:1-15. doi: 10.3389/fninf.2013.00050.
- Stoquart-ElSankari S, Lehmann P, Villette A, Czosnyka M, Meyer M-E, Deramond H, Balédent O. A Phase-Contrast MRI Study of Physiologic Cerebral Venous Flow. J. Cereb. Blood Flow Metab. 2009;29:1208–1215. doi: 10.1038/jcbfm.2009.29.
- Stuber M, Spiegel MA, Fischer SE, Scheidegger MB, Danias PG, Pedersen EM, Boesiger P. Single breath-hold slice-following CSPAMM myocardial tagging. Magn. Reson. Mater. Physics, Biol. Med. 1999;9:85–91. doi: 10.1016/S1352-8661(99)00049-6.
Figures
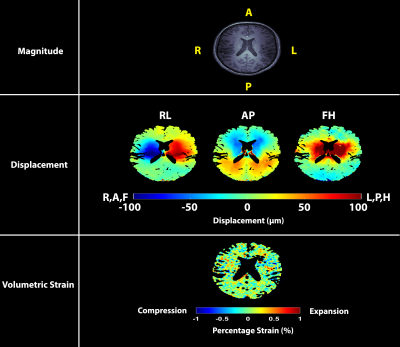
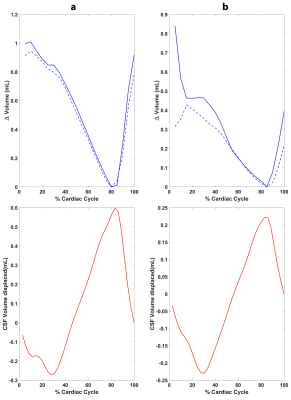
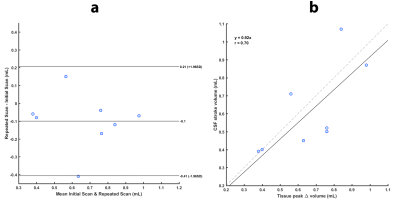
Table 1. Imaging parameters used in the study.
- The flip angles were varied over the cardiac cycle to create a stable signal9, assuming a T1 of 1100 ms.
- The retrospectively gated acquisitions were performed using a pulse oximeter as the triggering device, which was attached to the left index finger.
- Velocity was measured in the FH direction. One subject was scanned with a Venc = 10 cm/s to eliminate phase wraps.
- Scan duration reported for a heart rate of 60 beats/min, where applicable.