0312
Imaging of the Thoracic Spinal Cord using Radially Sampled Averaged Magnetization Inversion Recovery Acquisitions (rAMIRA)Matthias Weigel1,2, Tanja Haas1,3, and Oliver Bieri1,2
1Division of Radiological Physics, Dept. of Radiology, University Hospital Basel, Basel, Switzerland, 2Dept. of Biomedical Engineering, University of Basel, Basel, Switzerland, 3Dept. of Radiology, University Hospital Basel, Basel, Switzerland
Synopsis
Particularly for improved imaging of the thoracic spinal cord, a radial sampling variant of the averaged magnetization inversion recovery acquisitions approach was developed (radial AMIRA). The radial AMIRA approach is less prone to fold over artifacts and it is demonstrated that simple pulse triggering removes potential streak artifacts that may occur near big vessels or near the heart. Just as for the conventional Cartesian AMIRA approach, a series of images with remarkable different tissue contrasts is acquired and some of these images can be combined to enhance and fine-tune the desired tissue contrast.
Introduction
Magnetic Resonance Imaging of the spinal cord (SC) has gotten a great deal of attention in the past years.1,2 Recently, a novel SC imaging approach termed Averaged Magnetization Inversion Recovery Acquisitions (AMIRA) was suggested.3 Within a single measurement, AMIRA acquires a series of images with different tissue contrasts after an inversion recovery (IR) preparation. These images can be combined to further improve the contrast to noise ratio (CNR) between gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF).3A further development of the AMIRA approach that utilizes a radial balanced steady state free precession (bSSFP) based readout (rAMIRA) is presented. rAMIRA was particularly designed for thoracic SC imaging, since it is less prone to fold over artifacts. The present work also investigates further issues to improve rAMIRA’s image quality and demonstrates that the rAMIRA approach allows successful SC imaging even in the difficult parts of the thoracic SC.
Methods
The rAMIRA approach, being an advancement of the basic AMIRA approach,3 is based on an IR prepared, segmented cine-type bSSFP sequence as depicted in Figure 1. For rAMIRA, the bSSFP readout samples k-space with linearly increasing, segmented radial projections. After a non-selective adiabatic inversion of magnetization, five sample images with the effective inversion times TIeff = [174; 239; 304; 368; 433]ms are acquired in 52 shots (averages = 2). The bSSFP sequence parameters were: FOV = 128x128mm2, radial k-space sampling with 256 samples and 260 projections, 10 segments, resulting isotropic in-plane resolution 0.50 x 0.50 mm2, TRbSSFP = 6.49ms, TE = 3.23ms, flip angle = 50deg, bandwidth = 310Hz/Px. Transversal slices of thickness 8mm orientated orthogonal to the course of the spinal cord were measured at the thoracic SC.Apart from the thoracic “standard protocol” described above, additional rAMIRA examinations with a lower in-plane resolution of 0.67 x 0.67 mm2 (192 samples, 264 projections, 11 segments) and a very high in-plane resolution of 0.36 x 0.36 mm2 (352 samples, 352 projections, 8 segments) were carried out.
To avoid potential streak artifacts in the images, the rAMIRA measurements were synchronized with the patient’s heart beat (simple pulse triggering via an infrared finger clip). In dependence on the patient’s heart frequency, the rAMIRA sequence only triggered on every 3rd or 4th heart beat such that a minimum rest time of ca. 2.3s was ensured, which corresponds to a minimal TRIR-IR of 2.8s (Figure 1). The resulting acquisition time was approximately 2:36min based on a heart rate of 60bpm, for instance. A magnitude reconstruction was used for all images.
All experiments were performed on a 3T whole-body MR system using the elements of the built-in spine coil for RF reception.
Results
Figure 2 compares two representative series of the five inversion images acquired at the “standard” in-plane resolution of 0.50mm, one with and one without pulse triggering. In cases were acquisitions are near the heart or big vessels, streak artifacts can occur (upper row). These situations are also patient dependent. By introducing simple pulse triggering, such potential artifacts can be efficiently removed (cf. Figure 2).Figure 3 demonstrates that the acquired images display a remarkable tissue contrast variation for the SC that is inherent to the AMIRA approach in general.3 Particularly in the first image, the GM “butterfly structure” seems to virtually light up. For the rAMIRA technique, combining the first four acquired images seems recommendable (subfigure "Mean").
Figure 4 shows the practicability of the rAMIRA approach for several locations along the thoracic spine.
Finally, Figure 5 further indicates the potential and feasibility of acquiring very high 0.36mm in-plane resolutions at 8 mm slice thickness intentionally performed in a comparatively fast acquisition time of 4:24 min. Furthermore, a lower 0.67mm in-plane resolution is depicted for comparison.
Discussion and Conclusion
The suggested rAMIRA approach simultaneously acquires five images of noteworthy different tissue contrast with a 0.50 x 0.50 x 8.0mm3 resolution in typically 2:36min. The chosen radial k-space sampling strategy is particularly beneficial for thoracic SC imaging, since fold over artifacts are efficiently avoided by the inherently doubled FOV. Potential streak artifacts are also avoided by introducing simple pulse triggering. This concept ensures that the radial k-space lines in different segments are always acquired with the same phase of the heartbeat. rAMIRA, being a radial sampling technique, may be also advantageous in terms of inherent motional averaging. This aspect is a matter of further investigation. As an additional benefit that is common to all the AMIRA approaches, the rAMIRA images can be combined to further improve the contrast of the SC tissues.Acknowledgements
This work was funded by the Swiss National Science Foundation (SNF), grant number 320030_156860.References
- Wheeler-Kingshott CA, Stroman PW, Schwab JM et al. The current state-of-the-art of spinal cord imaging: Applications. Neuroimage 2014;84:1082-1093.
- Stroman PW, Wheeler-Kingshott C, Bacon M et al. The current state-of-the-art of spinal cord imaging: Methods. Neuroimage 2014;84:1070-1081.
- Weigel M, Bieri O. Spinal cord imaging using averaged magnetization inversion recovery acquisitions. Magn Reson Med 2017 Jul 16. doi: 10.1002/mrm.26833.
Figures

Figure 1. Outline of the suggested multi-shot IR prepared bSSFP sequence with a time-limited cine sampling, which is based on the published AMIRA principle.3 For the first 0.5s after the inversion pulse (acquisition window), segmented k-space data are radially acquired for five images of different effective TI. The inversion pulses are synchronized with the patient’s heart beat such that a minimal rest time of approx. 2.3s is ensured for relaxation after the acquisition window. For the typical rAMIRA protocol presented here, 52 shots shot are needed for acquisition (averages = 2). This corresponds to a typical acquisition time of ca. 2:36min per slice.
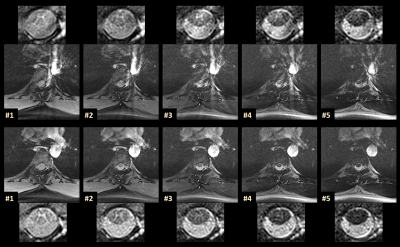
Figure 2. Comparison of rAMIRA inversion images acquired without (upper row) and with (lower row) pulse triggering at the location TH4-5 (“standard protocol”). Both the full rAMIRA images and the close-up views of the SC are shown. In unfavorable situations as displayed here, streak artifacts can be generated by the radial acquisition / reconstruction due to the proximity to flow in big vessels or to the heart. Introducing a synchronization with the heart beat via pulse triggering successfully removes these potential streak artifacts in the rAMIRA sequence (cf. Figure 1).
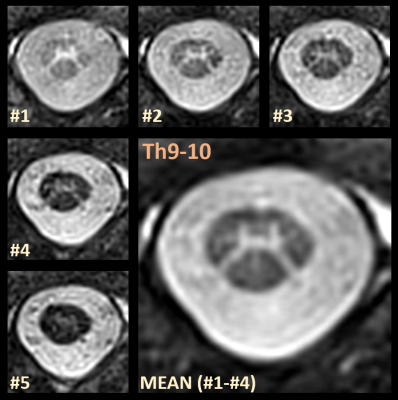
Figure 3. Representative example for the five images acquired at the level of the spinal disc Th9-10 with the suggested rAMIRA approach. The numbered images are shown in chronological order from lowest to highest TIeff. In accordance with the fundamental AMIRA approach,3 image #1 displays the highest GM-WM contrast, whereas image #5 displays the highest WM-CSF contrast. (Some of) the inversion images are combined to enhance the desired tissue contrast. Here, image #1 up to image #4 were averaged to obtain a SC image of improved quality and enhanced GM to WM contrast to noise (enlarged figure).
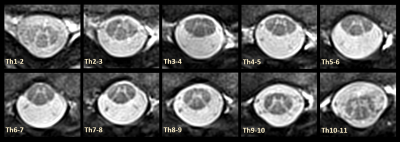
Figure 4. A series of cross-sectional rAMIRA measurements along the thoracic SC is presented. Despite the rather difficult MR imaging conditions particularly in the upper part of the thoracic SC, the rAMIRA approach yields a really good image quality. To note, an in-plane resolution of 0.50mm is definitely necessitated for such fine structures in the thoracic SC and for this reason it is also referred to as being the “standard protocol” within this work.
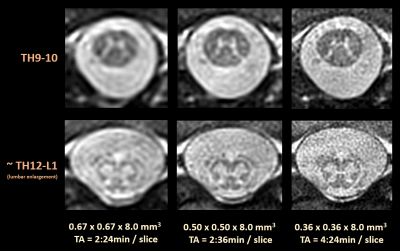
Figure 5. Compilation of rAMIRA images of different in-plane resolution near the end of the SC as specified. Owing to the difficult RF reception conditions - only one RF spine coil element can be used for RF reception -, the very high in-plane resolution of 0.36mm does not yield sufficient signal-to-noise, although it appears to be really valuable.