0308
Robust estimation of quantitative perfusion from multi-phase pseudo-continuous arterial spin labelling1Institute of Biomedical Engineering, University of Oxford, Oxford, United Kingdom, 2Cancer Research UK & Medical Research Council Oxford Institute for Radiation Oncology, Department of Oncology, University of Oxford, Oxford, United Kingdom, 3Wellcome Centre for Integrative Neuroimaging, FMRIB, University Oxford, Oxford, United Kingdom
Synopsis
Multi-phase pcASL has been proposed as a means to achieve accurate perfusion quantification that is robust to imperfect shim in the labelling plane. There exists a previously unrecognised bias in the estimation process that is a function of noise on the data. In this work this boas is addressed, exploiting information common to voxels containing tissue fed by the same artery, identified using clustering methods.
Introduction and Purpose
A potential short-coming for pseudo-continous ASL (pcASL) perfusion imaging is the sensitivity of the labelling to phase mismatch due to off-resonance fields and arterial flow velocity. One solution is to acquire so-called multi-phase pcASL data (MPpcASL), where labelling is performed at a range of phase offsets and subsequent model fitting used to extract the magnitude associated with the delivery of labeled blood1 and hence calculate perfusion. Alternatively the information gained from a multi-phase prescan can be used to adjust the labelling for a subsequent pcASL acquisition2.
A previously unrecognised issue for multi-phase approaches to pcASL is that the model-fitting process is biased by the appearance of noise on the data. In practice, the SNR of ASL data is insufficient to generate unbiased measurements of phase-offset in the labeled arteries and thus of perfusion on a voxelwise basis. The purpose of this study was to address this issue, exploiting the fact that in reality we only need to determine phase offset for each labeled artery and thus can exploit common information found in multiple voxels within a whole region of the brain, offering superior SNR and thus overcoming the bias.
Methods
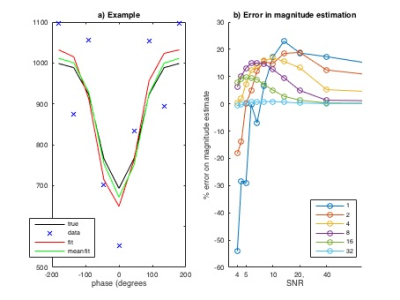
Simulation study: Data were simulated according to the modified Fermi function1 at 8 phase offsets equally spaced across the full 360 degrees, adding white noise at varying SNR (defined relative to the offset, i.e. static tissue, magnitude) generating 40 instances at each SNR, and then fitted to the same modified Fermi function with three variables (magnitude, phase and offset) using a variational Bayesian model fitting routine3,4,5.
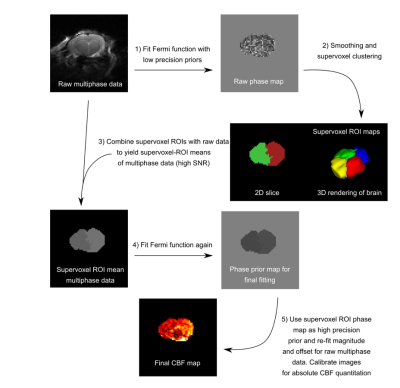
Multi-stage analysis solution: Figure 1 illustrates the multi-stage procedure proposed to overcome the bias observed in the model-fitting procedure for MPpcASL data. The main features are: (1) an initial voxelwise fit to produce biased maps of the parameters, (2,3) a supervoxel based clustering procedure6 on the (biased) phase map to indetify regions of common phase, (4) model-fitting of the average series in each cluster, (5) additional voxelwise model-fitting with the phase parameter fixed by the appropriate value from the cluster analysis. The key concept is that the phase parameter is specific to the feeding arteries and thus is common across a large number of voxels, appropriate regions of interest can be identified using the initial (biased) phase maps to separate regions with different phase (and thus potentially different feeding artery), and averaging of data within an ROI results in data with a higher SNR, and thus reduced bias when estimating the phase parameter. The multi-stage procedure was implemented in Quantiphyse7.
Data: MPpcASL was acquired in isoflurane anaesthetised female Wistar, Sprague Dawley rats (n=3) at 9.4 T (Agilent). The sequence used eight phase angles, with optimal label duration of 1.4 s and post-label delay of 0.55 s and labeling plane positioning following8. A multislice single-shot spin echo EPI sequence was used for the imaging readout, with a 32x32 mm FOV (64x64 matrix, thickness=1 mm, 10 slices), TE=28.7 ms. For validation perfusion was also determined using autoradiography as in8.
Evaluation and validation: Perfusion was quantified using both conventional MPpcASL modified-Fermi fitting and the multi-stage procedure proposed. Kinetic model inversion was performed using BASIL from FSL5. Absolute perfusion values were derived by estimating the equilibrium magnetisation in striatum and converting to the equivalent value in blood based on the partition co-efficient8. These values were compared to the equivalent measure derived from autoradiography.
Results
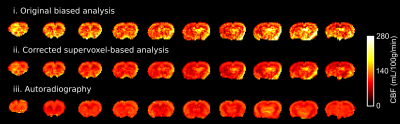
Figure 2 illustrates the extent of bias observed when fitting a modified fermi function to multi-phase pcASL data using simulated data. As the SNR decreases there is a systematic bias introduced in the fitted parameters, which would translate to an error in perfusion at typical SNR. Figure 3 compares the quantified perfusion from conventional MPpcASL analysis and the multi-stage solution and autoradiography. Autoradiography CBF: 116±14 mL/100g/min; Original, biased CBF: 136 ± 27 mL/100g/min; New, multi-stage CBF: 111±18 mL/100g/min. One-way ANOVA p<0.05, Newman-Keuls post-hoc: multi-stage and autoradiography, both p<0.05 vs biased CBF.Discussion and Conclusion
By adopting a multi-stage procedure incorporating an automated clustering to generate high SNR ROIs, a previously unreported bias in perfusion quantification using MPpcASL has been addressed. This methodology could be extended to a more accurate model of the variation in the MPpcASL signal with phase offset that includes flow velocity, such as been exploited in vessel-encoded ASL analysis9, potentially allowing for territory specific correction for labelling efficiency without needing artery selective ASL acquisition. In principle the clusters generated as part of the multi-stage procedure could offer information about flow territories, although this would require further validation.Acknowledgements
This work was funded by the CRUK/EPSRC Cancer Imaging Centre (C5255/A16466) in Oxford, Cancer Research UK (C5255/A15935), and the EPSRC (EP/P012361/1).References
[1] Jung, Y., Wong, E. C., & Liu, T. T. (2010). Multiphase pseudocontinuous arterial spin labeling (MP-PCASL) for robust quantification of cerebral blood flow. Magnetic Resonance in Medicine, 64(3), 799–810. http://doi.org/10.1002/mrm.22465
[2] Hirschler, L., Debacker, C. S., Voiron, J., Köhler, S., Warnking, J. M., & Barbier, E. L. (2017). Interpulse phase corrections for unbalanced pseudo‐continuous arterial spin labeling at high magnetic field. Magnetic Resonance in Medicine, 60, 1488. http://doi.org/10.1002/mrm.26767.
[3] Chappell, M. A., Groves, A., Whitcher, B., & Woolrich, M. (2009). Variational Bayesian Inference for a Nonlinear Forward Model. IEEE Transactions on Signal Processing, 57(1), 223–236.
[4] Shin, D. D., Chappell, M. A., & Liu, T. T. (2014). Robust and Fast Quantification of CBF measures for Multiphase PCASL using Bayesian Nonlinear Model Fitting (p. 2176). Presented at the Proc. Int. Soc. Magn. Reson. Med., Milan.
[5] www.fmrib.ox.ac.uk/fsl/basil
[6] Irving, B., Popescu, I. A., Bates, R., Allen, P. D., Gomes, A. L., Kannan, P., et al. maskSLIC: Regional Superpixel Generation withApplication to Local Pathology Characterisation inMedical Images. arXiv.org. http://doi.org/arXiv:1606.09518
[7] www.quantiphyse.org
[8] Simard, M. A., Larkin, J. R., Khrapitchev, A. A., Meakin, J. A., Okell, T. W., Jezzard, P., et al. (2016). Validation of quantitative pre-clinical pseudo-continuous ASL in rat brain (p. 1891). Presented at the International Society for Magnetic Resonance in Medicine, Honolulu, USA.
[9] Chappell, M. A., Okell, T. W., Payne, S. J., Jezzard, P., & Woolrich, M. W. (2012). A fast analysis method for non-invasive imaging of blood flow in individual cerebral arteries using vessel-encoded arterial spin labelling angiography. Medical Image Analysis, 16(4), 831–839. http://doi.org/10.1016/j.media.2011.12.004
Figures