0302
Advanced Automatic Planning for Super-Selective Arterial Spin Labeling Flow Territory MappingMichael Helle1, Fabian Wenzel1, Kim van de Ven2, and Peter Boernert1
1Philips Research, Hamburg, Germany, 2Philips Healthcare, Best, Netherlands
Synopsis
This study presents an advanced fully automated approach based on vessel detection and analysis. It is completely integrated in the scanner console and allows labeling of the major brain feeding vessels in an efficient and robust way. Average processing time to find optimal labeling positions for all major brain feeding arteries is <15 seconds.
Introduction
Super-Selective Arterial Spin Labeling (ASL) proved to be an efficient method for selective labeling of individual blood vessels (1). It can be used in conjunction with different acquisition modules, thereby, generating angiograms of the cerebral vasculature (2) or perfusion territory maps of the brain (3) and has been applied in various patient studies (4, 5). However, careful planning of the labeling spot is required and might add extra time to the imaging protocol. Recently, first attempts to facilitate the positioning of the labeling spot have been introduced and compared to images of manually planned labeling spots (6, 7). This study presents an advanced fully automated approach based on vessel detection and analysis. It is completely integrated in the scanner console and allows labeling of the major brain feeding vessels in an efficient and robust way.Methods
Measurements were performed in six healthy volunteers on a 1.5T Ingenia Scanner (Philips, Best, The Netherlands) using a 16-element head-coil. Automatic planning of the labeling spots was performed on the basis of a time-of-flight (TOF) scan to visualize the vascular anatomy of the neck (FOV 200x200x96mm3, voxel size 1.5x1.5x1.5mm3, 3D fast-field echo acquisition, FA 18°,TR/TE 23/2.3ms, 1:07min scan time). The automated planning consists of a sequence of image processing steps, including vessel tracking functionality from a commercial software package (6, 8). Parameters with impact on the final labeling spot of a particular vessel are based on three rejection criteria: First, the angular deviation from the z-direction of the magnet. Second, the extent of the labeling plane into which the course of a vessel should not re-enter after labeling. Third, the maximum length of a vessel part inside the labeling plane. The present implementation allows a maximum angular deviation of 5° in order to reduce possible effects of gradient inhomogeneities to the labeling efficiency. A width of the labeling plane has been set to w=18mm, approximating its true width according to the applied labeling gradient in z-direction. A maximum length of a vessel part inside the labeling plane has been set to l=22mm, therefore excluding labeling positions of highly curved, and unsuitably elongated vessel parts. More parameter combinations (w=18-25mm; l=22-27mm) and their impact on labeling locations were investigated on 10 retrospective TOF acquisitions. Image acquisition for flow territory mapping was performed based on the automatic positioning of the labeling spots onto both internal carotid arteries (ICA) and both vertebral arteries (VA). Scan parameters for super-selective ASL were: 2-pulse background suppression, segmented 3D GraSE read-out (FOV 240x240x112mm3, voxel size 3.75x3.75x8mm3, FA 90°, TSE/EPI factor 17/15, TR/TE 3913/15ms, 3 averages, labeling duration 1.8s, post-labeling delay 1.8s; 1:57min scan time per vessel). Subsequently, the labeling efficiency was calculated by normalizing the signal intensities of flow territory images with respect to the signal intensity of an additionally performed non-selective ASL scan.Results
Figure 1 presents the angiogram and analyzed vessel architecture with calculated positions of the labeling spots based on two different tracking parameter settings for one single volunteer. Labeling was effective for each parameter setting with no significant change in image quality, even though the labeling spots for the right ICA and VA were in a different position. Visual inspection of results from altered tracking parameters has confirmed suitability of this choice and has led to more configurations with no valid labeling focus otherwise. Average processing time in each volunteer to find optimal labeling positions for all major brain feeding arteries was approximately 12s. Successful flow territory mapping was performed (figure 2) and similar labeling efficiency was achieved in the ICAs when compared to a non-selective ASL scan (figure 3). However, labeling efficiency decreased in VAs possibly due to mixing of the blood in the posterior circulation. In three volunteers, only the signal of one VA was detected as the contralateral VA presented tiny with small caliber and probably only has minor contribution to the perfusion of the posterior circulation (figure 1a, 2).Discussion
Fully automatic positioning of the labeling spot can be helpful in clinical routine scan protocols to overcome user-dependent and time-consuming planning. The presented approach demonstrates fast and reliable planning; slight changes of the tracking parameters may shift the positioning of the labeling spot in some vessels, but the differences seem too small to have a significant impact on image quality. However, this might be different in patients with altered vasculatures and would require separate investigation. The planning algorithm presented stable even in small caliber arteries like some of the VAs, however, as the amount of labeled blood can be too small to generate detectable signal in the brain, instead, one may consider using elliptical labeling spots to label both VAs at the same time (9).Acknowledgements
No acknowledgement found.References
- Helle M, Norris DG, Rufer S, Alfke K, Jansen O, van Osch MJP. Superselective pseudocontinuous arterial spin labeling. Magn Reson Med 2010;64:777-786.
- Jensen-Kondering U, Lindner T, van Osch MJ, Rohr A, Jansen O, Helle M. Superselective pseudo-continuous arterial spin labeling angiography. Eur J Radiol 2015;84:1758-67.
- Hartkamp NS, Helle M, Chappell MA, Okell TW, Hendrikse J, Bokkers RP, van Osch MJ. Validation of planning-free vessel-encoded pseudo-continuous arterial spin labeling MR imaging as territorial-ASL strategy by comparison to super-selective p-CASL MRI. Magn Reson Med 2014;71:2059-70.
- Helle M, Rüfer S, van Osch MJ, Nabavi A, Alfke K, Norris DG, Jansen O. Superselective arterial spin labeling applied for flow territory mapping in various cerebrovascular diseases. J Magn Reson Imaging. 2013;38:496-503.
- Richter V, Helle M, van Osch MJP, Lindner T, Gersing AS, Tsantilas P, Eckstein HH, Preibisch C, Zimmer C. MR
Imaging of Individual Perfusion Reorganization Using Superselective
Pseudocontinuous Arterial Spin-Labeling in Patients with Complex
Extracranial Steno-Occlusive Disease. AJNR Am J Neuroradiol. 2017;38:703-711.
- Helle M, van de Ven K, Wenzel F. Automatic Planning for fast and robust Flow Territory Mapping. Proc. Int. Soc. Magn. Reson. Med. 2017:3625.
- Lindner T, Jansen O, Helle M. Hough-transform based detection of vascular structures applied to automate and accelerate planning of super-selective Arterial Spin Labeling. Proc. Int. Soc. Magn. Reson. Med. 2017:3823.
- Bescós J, Sonnemans J, Habets R, Peters J, van den Bosch H, Leiner T. Vessel Explorer: A tool for quantitative measurements in CT and MR angiography. Medicamundi 2009:53/3.
- Helle M, Rüfer S, van Osch MJ, Jansen O, Norris DG. Selective multivessel labeling approach for perfusion territory imaging in pseudo-continuous arterial spin labeling. Magn Reson Med 2012;68:214-9.
Figures
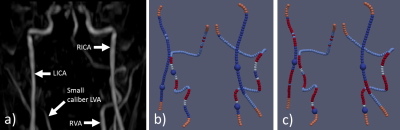
Figure
1 (Vessel analysis and planned labeling spots): Maximum-Intensity-Projection
of a TOF scan (a) and analysed vessel architecture with detected labeling spots
(large blue spheres) according to different tracking parameters (b, c). Colors
represent rejection/acceptance criteria. Orange: Rejected, no curvature information
due to proximity to FOV. Light blue: Rejected, angle to z-direction larger than
threshold. White: Rejected, vessel impinges labeling plane. Red: Rejected,
vessel segment in labeling plane longer than threshold l. Dark blue: Accepted.
Proposed rejection parameters for tracking w=18, l=25 (b) and w=20, l=25 (c). Efficient labeling was achieved for each
parameter setting, nonetheless the changes in location between the two settings.
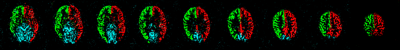
Figure
2 (Flow territory maps): Example images of one volunteer to demonstrate
successful flow territory mapping of right (green) and left (red) ICAs and left
VA (blue). The right VA presented tiny with small caliber and has no
significant contribution to the perfusion of the posterior circulation.
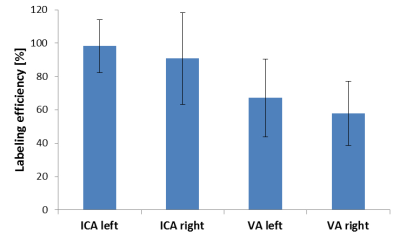
Figure
3 (Quantitative analysis): Similar labeling efficiencies can be achieved with
automatic planning in the ICAs when compared to a non-selective ASL scan (signal
intensities of flow territory images were normalized to the signal intensity of
a non-selective ASL scan). Decreased labeling efficiency in VAs possibly presents
due to mixing of the blood with the non-labeled contralateral VA after merging
in the basilar artery.