0298
Magnetic resonance imaging in patients with cardiac implanted electrical devices: single centre two year experience including thoracic imaging and non-MRI conditional devices.1Clinical Physics, Barts Health NHS Trust, London, United Kingdom, 2Medical Physics and Engineering, Kings College London, London, United Kingdom, 3Department of Cardiovascular Imaging, Barts Heart Centre, Barts Health NHS Trust, London, United Kingdom, 4Institutes for Cardiovascular Science, University College London, London, United Kingdom, 5National Institutes of Health, Bethesda, MD, United States, 6William Harvey Research Institute, Queen Mary University of London, London, United Kingdom
Synopsis
Over a million patients worldwide have cardiac implantable electronic devices (CIEDs), with a 50-75% lifetime MRI requirement. Although conventionally contraindicated, MRI-conditional CIEDs and evidence supporting safer scanning of non-MRI conditional CIEDs are changing practice. We report single center experience of CIED MRI scanning over 24 months. 179 MRI scans were acquired, 31% non-MRI conditional devices, 79% thoracic scans. Clinical impact was high (including cancer diagnosis and treatment planning, suspected cord compression and stroke). All patients were safely scanned with no clinically-significant events or device parameter changes resulting from MRI. These data support increased provision of MRI to CIED patients.
INTRODUCTION
Historically, MRI was contraindicated for patients with cardiac implanted electrical devices (CIEDs) including permanent pacemakers (PPMs) and implantable cardiac defibrillators (ICDs), due to safety concerns.1 With increasing frequency of CIED implantation and expanding indications for MRI, the clinical requirement for MRI in device patients is rising exponentially. For many indications, particularly within the domains of oncology and neurology, alternative imaging modalities are inferior or non-viable. 2-4. Development of MRI conditional CIEDs has partially addressed this problem, but guidelines5,6,7 still recommend safety protocols addressing scanner and device parameters, as well as exclusion zones. Unfortunately this has discouraged many centres from providing MR imaging even to this cohort of patients with MRI conditional CIEDs. Concurrently, there is increasing evidence that the risks of non-thoracic MR imaging in patients with legacy non-MRI conditional CIEDs may be overstated provided appropriate safety protocols are followed, and where there is clear clinical benefit to mitigate the small potential risk.5,8 Other smaller studies9,10 have suggested that thoracic MRI scanning in CIED patients may also be safer than previously thought. Our centre (UK specialist cardiac and cancer referral centre) has developed local protocols for MRI in CIED patients, and we sought to investigate the safety and feasibility of performing MRI scans in a tertiary referral centre population with high volumes of thoracic MRI scans and patients with non- conditional CIED.METHODS
Consecutive patients referred for MRI for clinical indications from October 2015 to October 2017 were included in the study. Local protocols based on ESC guidelines5 and Magnasafe Registry8 protocols were applied. All scans were performed on a 1.5T Aera scanner (Siemens Healthcare, Erlangen, Germany) operating in Normal Operating Mode (whole body SAR limit < 2 W/kg). Referrals were reviewed and details of the CIED system checked to assess for MRI conditionality before booking. For non-MRI conditional devices, referrals were reviewed for suitability (would the scan change management and could another imaging modality answer the clinical question?) and patients consented in writing to undergoing MRI. Patient monitoring was via continuous ECG and pulse oximetry, and an external defibrillator with pacing capability was available in the unit. MR conditional CIEDs were re-programmed according to manufacturer instructions pre and post scan. Non-MRI conditional devices were re-programmed in line with the ESC and Magnasafe recommendations, with an IBHRE accredited cardiologist was present in the department throughout. Endpoints recorded were clinical complications, changes to device parameters (using Magnasafe criteria8) and scan outcomes.RESULTS
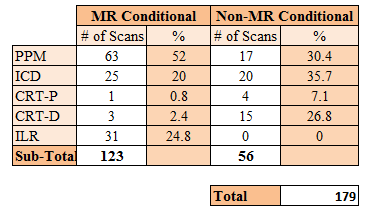
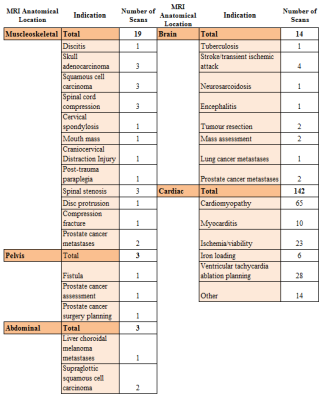
179 scans in 171 patients were performed within the 24 month period (table 1), 68% male (age range 18 to 88 years). Four requests were rejected (one abdominal pacing system, two where alternative imaging modalities could answer the clinical question in patients with non-MRI conditional devices, and one where the scan would not change clinical management). 68.7% of scans were for patients with MRI conditional devices (52.0% PPM, 20.0% ICD, 3.2% CRTP/D, 24.8% ILR) with the remaining 31.3% having non-MRI conditional devices (62.5% ICD/ CRTD). The majority (79.3 %) of requests (see table 2) were for cardiac scans, with extracardiac MRI indications including suspected spinal cord compression (n=3), surgical planning (n=1) and brain imaging for cerebrovascular accidents (n=4). There were no clinical complications (symptoms or clinical events) related to MRI. One patient with known paroxysmal atrial fibrillation had a self-terminating episode peri-scan, and one patient had a vasovagal on de-cannulation. Although 28 subjects had minor lead parameter changes post scan4, all had normalised or were stable on 3 month follow-up review. Diagnostic scans were obtained in all but two defibrillator patients undergoing CMR, where artefact from the generator prevented interpretation.DISCUSSION
This large single-centre experience
suggests that MRI in patients with CIEDs is both safe and feasible, including
for thoracic imaging and for patients with non-MRI conditional CIEDs. Scan indications were generally for conditions
where alternative imaging modalities were suboptimal, with important clinical
sequelae (cancer diagnosis and management, stroke diagnosis etc). Given that
over a million people worldwide have CIED implanted, with a lifetime
requirement for MRI of 50-75%, improving MRI provision to this cohort is
important.CONCLUSION
This data, alongside work from other groups, provides reassurance to expand provision of MRI in patients with CIEDs, to ensure that patients are able to access optimal imaging to enable timely diagnosis and appropriate clinical management.Acknowledgements
No acknowledgement found.References
[1] Kalin, R. and Stanton, M.S., 2005. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing and clinical electrophysiology, 28(4), pp.326-328.
[2] Saifuddin, A., 2001. MRI of acute spinal trauma. Skeletal radiology, 30(5), pp.237-246.
[3] Wintermark, Max, et al. "Imaging recommendations for acute stroke and transient ischemic attack patients: a joint statement by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery." American Journal of Neuroradiology 34.11 (2013): E117-E127.
[4] Türkbey, Bariş, et al. "The role of dynamic contrast-enhanced MRI in cancer diagnosis and treatment." Diagnostic and interventional radiology (Ankara, Turkey) 16.3 (2010): 186.
[5] Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association . Eur Heart J. 2013;34(29):2281-2329. doi:10.1093/eurheartj/eht150.
[6] Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Hear Rhythm. 2017;(May). doi:10.1016/j.hrthm.2017.04.025.
[7] Lowe MD, Plummer CJ, Manisty CH, et al Safe use of MRI in people with cardiac implantable electronic devices Heart Published Online First: 29 September 2015. doi: 10.1136/heartjnl-2015-308495
[8] Russo RJ, Costa HS, Silva PD, et al. Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. N Engl J Med. 2017;376(8):755-764. doi:10.1056/NEJMoa1603265.
[9] Chow, G.V. and Nazarian, S., 2014. Magnetic resonance imaging for patients with cardiac implantable electrical devices. Cardiology clinics, 32(2), p.299.
[10] Horwood, L., Attili, A., Luba, F., Ibrahim, E.S.H., Parmar, H., Stojanovska, J., Gadoth-Goodman, S., Fette, C., Oral, H. and Bogun, F., 2016. Magnetic resonance imaging in patients with cardiac implanted electronic devices: focus on contraindications to magnetic resonance imaging protocols. Europace, 19(5), pp.812-817.
Figures