0294
NEUROMAN: Reference Computational Human Phantoms for Evaluation of Safety Thresholds for Peripheral Nerve StimulationBryn A Lloyd1, Antonino Cassarà1, Silvia Farcito1, Esra Neufeld1, Beom Sun Chung2, Jin Seo Park3, Min Suk Chung2, and Niels Kuster1,4
1IT'IS Foundation, Zürich, Switzerland, 2Department of Anatomy, Ajou University, Suwon, Republic of Korea, 3Department of Anatomy, Dongguk University, Gyeongju, Republic of Korea, 4ETH, Zürich, Switzerland
Synopsis
The trend towards stronger magnetic fields and/or faster gradient switching in magnetic resonance imaging poses safety risks for patients, e.g., due to tissue heating and unwanted neurostimulation. The IEEE-ICES TC95 SC6 was formed to re-evaluate nerve excitation safety thresholds in response to temporal and spatial characteristics of electric fields induced by externally applied fields or implants. To this end, we are developing reference human anatomical models with unprecedented details in the peripheral nervous system, connectivity to organs and muscles, and functionalized with compartmental nerve models to investigate interactions with neuronal electrophysiology. We employ these phantoms to investigate current safety guidelines.
Introduction
Magnetic resonance imaging (MRI) is a powerful, non-invasive diagnostic technique routinely used in clinical settings and research. While stronger magnetic fields and faster, more intense gradient switching could improve image resolution or functional contrast, in many MRI-based technologies (e.g., functional MRI, diffusion tensor imaging, etc.), there are accompanying potential risks of radiofrequency (RF) tissue heating and unwanted peripheral nerve stimulation (PNS). These risks are even greater in subjects wearing metallic implants. While computational techniques to quantify RF exposure, power absorption, and thermal consequences are well established1,2 and regulated by safety standards and guidelines3-7, predictive tools to study nerve electrophysiology within the complex human anatomical environment and estimate the risk of PNS are lacking. We previously developed the concept of neuro-functionalized models8 that includes populations of axons geometrically represented as splines embedded in the human anatomy. Electrophysiological models (e.g., SENN, Sweeney, etc.) assigned to the spline trajectories are used to predict the responses of axons and nerves to electromagnetic stimuli. Embedding the nerve models in the anatomy facilitates coupled simulations of the neuro-physiology with induced electrical fields and non-trivial field gradients that result from the complex dielectric material distributions inside the body. This methodology has been successfully applied in different contexts, e.g., the development of electroceuticals9, in investigations concerning non-invasive brain stimulation10, concerning the risk of unwanted PNS by MRI gradient switching11, and in studies aimed at critical revision of current safety standards12. These investigations provide insight into principal mechanisms and causes of neurostimulation and largely reproduce experimental findings. However, the lack of realistic anatomical models with detailed nerve trajectories extracted from 3D image data prevents quantitative prediction in the context of PNS in MRI. To overcome these limitations and provide solutions to mitigate PNS, we initiated the NEUROMAN project, in which we aim to create two reference high-resolution neuro-functionalized human anatomical models. The two phantoms, a female and a male, include detailed mapping of nerve trajectories of the peripheral nervous system, from the cranium and spinal cord to internal organs and all major muscles of the human body.Methods
The Visible Korean Human13 male (33 y, 1.64 m, 55 kg) and female (26 y, 1.52 m, 55 kg) cryosection data are being used as the basis for the new phantoms due to the unique resolution (0.2 × 0.2 × 0.2 mm) and quality of these images. To segment important peripheral nerves, a nerve tracing tool has been developed, which allows to semi-automatically extract smooth nerve trajectories by specifying sparse (start/end) points along a nerve in the cryosection image stack. Functionalization is achieved by assigning electrophysiological models of myelinated and unmyelinated axons to axon trajectories within nerve models based on histological investigations documented in the literature. The computational phantoms will continue evolving, but already over 900 different tissues and structures have been segmented, including more than 320 muscles. Following major nerves are or will soon be completed in the male model: the vagus nerve and other cranial nerves and the lumbar, brachial and sacral plexus. To ensure high quality standards, we follow an internal/external review approach similar to that of the Virtual Population models2.Results
Preliminary results on simplified implant leads and electrode geometries proximal to nerve structures (Fig. 3) with electrophysiological axonal models exposed to electric fields in the 10 – 100 kHz frequency range, demonstrate that neurostimulation and conduction blocking (CB) of nerve signals are possible risks even at exposure levels in compliance with standards3,4, due to simultaneous interference between pulsed MRI gradients and medical implants and field enhancement at lead electrodes. Effective exposure thresholds depend on the tissue material properties surrounding the nerve, implant trajectories and shape, pulse sequences and gradient coils. In current work, we explore the risk of PNS and CB near implants within anatomical bodies and for MRI relevant waveforms, e.g., 3D echo-planar imaging and spiral waveforms.Conclusion
The developed phantoms, which constitute an important expansion of the Virtual Population (www.itis.ethz.ch/vip), which includes reference models for a wide range of biomedical applications. The NEUROMAN models are expected to significantly impact the scientific community and the field of EM-neuron research to enable studies of multi-scale models with realistic anatomies and electrophysiology. The results will enhance our understanding of mechanisms of neurostimulation, e.g., in MRI, provide experimental test-beds for new therapeutic approaches and devices, and enable study of safety aspects, providing a tool to facilitate regulatory submissions and standardization activities.Acknowledgements
This research has received funding and support from the Swiss Commission for Technology and Innovation (CTI 25290.1 PFLS-LS) and the Korean Institute for Advancement of Technology.References
- Murbach M, Neufeld E, Kainz W et al. Whole-body and local RF absorption in human models as a function of anatomy and position within 1.5T MR body coil. Magn Reson Med 2014;71:839–845
- Gosselin M-C, Neufeld E, Moser H, et al. Development of a new generation of high-resolution anatomical models for medical device evaluation: the Virtual Population 3.0. Phys Med Biol 2014;59:5287–5303
- ICNIRP 1998 Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz) Health Phys. 74 494–521
- ICNIRP 2010 Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz) Health Phys. 99 818–36
- IEEE-C95.1 2006 IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz (New York: IEEE)
- IEEE-C95.6 2002 IEEE Standard for Safety Levels with Respect to Human Exposure to Electromagnetic Fields, 0 to 3 kHz (New York: IEEE)
- Reilly PJ, Hirata A, Low-frequency electrical dosimetry: research agenda of the IEEE International Committee on Electromagnetic Safety, Phys Med Biol 2016, 61(12):138
- Neufeld E, Cassarà AM, Montanaro H, Kuster N, Kainz W. Functionalized anatomical models for EM-neuron interaction modeling. Phys Med Biol 2016, 61(12):4390
- Cassarà AM, Neufeld E, Kuster N. Vagus Nerve Stimulation: Comparison of Thresholds for A-, B-and C-Fiber Recruitment for Cuff Electrodes and Alternative Stimulation Configurations in Functionalized Generic and Anatomical Models
- Cassarà AM, Neufeld E, Guidon M et al. Large-Scale Multi Neuronal Simulation within an Anatomical Head Model for Transcranial Alternative Current Stimulation (tACS) Investigations, Proc Joint Meeting of BEMS and EBEA 2016
- Cassarà AM, Neufeld E, Hagberg G et al. Peripheral Nerve Stimulation in MRI: Insights from a three level analysis and coupled EM-electrophysiological simulations in neuro-functionalized human models, Proc ISMRM 2017
- Neufeld E, Oikonomidis IV, Iacono MI, et al. Investigation of assumptions underlying current safety guidelines on EM-induced nerve stimulation. Phys Med Biol 2016, 61(12):4466
- Park JS, Chung MS, Hwang SB et al. Visible Korean human: improved serially sectioned images of the entire body. IEEE Trans Med Imaging 2005, 24(3):352-60
Figures
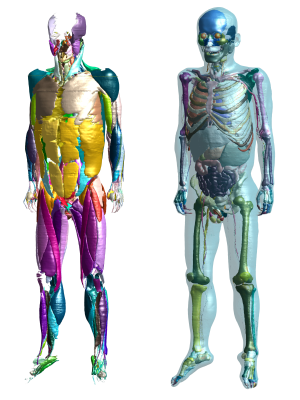
The male anatomical phantom already contains over 900 tissues
and structures, including more than 320 individual muscles. On the left only
the muscles are depicted. The right shows internal organs, bones and the skin
(transparent).
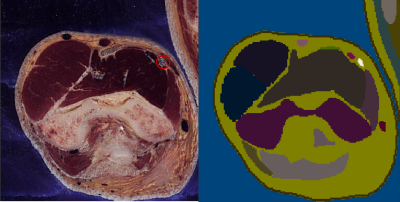
A slice through the male might arm with the median nerve
highlighted (red circle) in the cryosection image (left) and segmentation
(right).
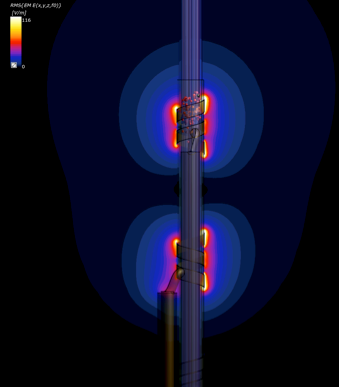
Model used to quantify exposure thresholds for activation (or conduction
blocking) of axons within a nerve surrounded by electrodes, as a consequence of
interaction between incident fields in the kHz frequency range and implants,
resulting in field enhancement around the electrodes. In this example, cuff
electrodes surround a model of a simplified vagus nerve featuring fascicles and
electrophysiological models of axons. The incident field is parallel to the
lead. Small red spheres indicate spiking neurons.