0292
Reduction of Peripheral Nerve Stimulation (PNS) using Pre-Excitation Targeting the Potassium System (PRE-TAPS)1Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 2Martinos Center for Biomedical Imaging, Dept. of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Department of Anesthesiology Mannheim, Heidelberg University, Mannheim, Germany, 5Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States
Synopsis
PNS has become the main limitation of fast MRI using current gradient hardware. We recently presented a novel pipeline to simulate magnetically induced PNS thresholds in arbitrary coil geometries. Now, we use this framework and detailed modeling of ion dynamics and Action Potential (AP) generation to test a new strategy for PNS reduction. The method (which we call “PRE-TAPS”) pre-saturates the nerve membrane by playing gradient pulses prior to the main imaging gradient. Our model suggests that this simple pulse-sequence modification could effectively increase PNS thresholds by up to 30%.
Target audience
MR safety researchers, MRI gradient designers, MRI sequence developersPurpose
PNS has become the main constraint for fast imaging with current MRI hardware. Rapid gradient switching induces electric fields in the body powerful enough to cause PNS. We recently presented a simulation framework including a full neurodynamic model1 to enable gradient designers to predict PNS in arbitrary coil geometries and thus guide the design process2,3,4. Monitoring the parameters of the neurodynamic model also provides insights into modulation of neuronal excitability and has empowered us to propose an alternative, non-hardware approach for reducing PNS, which we refer to as Pre-Excitation Targeting the Potassium System (“PRE-TAPS”). Here we present simulations supporting the ability of this method to lower PNS thresholds. The concept is also supported by clinical trials performed by Kapural et al.5 and Avendaño-Coy et al.6.Methods
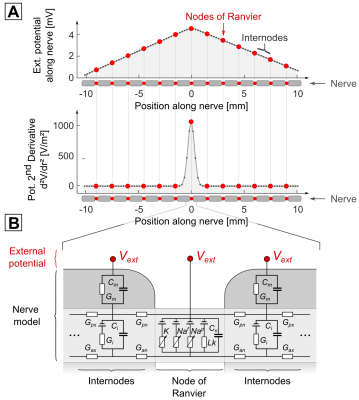
Neurodynamic Model: We used the MRG neurodynamic model1 to simulate the response of nerves to an imposed external electric potential (Fig. 1). The MRG model consists of an electrical circuit model with nodal and inter-nodal compartments for both the axon and the myelin insulation sheath and nodes of Ranvier. Every compartment is modeled as an RC circuit whose conductance/capacitance parameters match the electrical properties of human nerves. The circuits at the nodes of Ranvier are extended by voltage-dependent ionic conductances (for sodium, potassium, and non-specific ions) representing the membrane’s ion channels. The imposed electric potential varies spatially along the nerve, but also temporally following the applied gradient waveform. If the membrane is sufficiently charged by the external stimulus, the ionic permeabilities suddenly increase, creating a short but strong transmembrane current, an action potential (AP), and thus PNS. The stimulation threshold is found by repeating the simulation with increasing gradient amplitudes until an AP is noted (titration). Nerve Pre-Excitation: In addition to the MR imaging gradient under test, we add a short “pre-excitation” gradient waveform envisioned to occur before spin excitation without effect on the image, but potentially reducing axonal excitability. Because the same coil is used for pre-excitation and for the imaging sequence, the method is "spatially self-calibrated". In other words, the area where the pre-excitation is most effective is precisely the area where the coil creates PNS. We tested the ability of different pre-excitation gradients to increase PNS thresholds for a simple 1 kHz sinusoidal EPI imaging gradient (20 ms length). For the stimulated nerve fiber, we imposed a simple triangular potential pattern (e.g., resulting from an “L”-shaped nerve in a homogeneous E-field), that leads to a localized stimulus at only few nodes of Ranvier (at the kink of the triangular potential, Fig. 1). We computed PNS thresholds with and without a high-frequency 10 kHz pre-excitation gradient played out just before the imaging gradient.Results
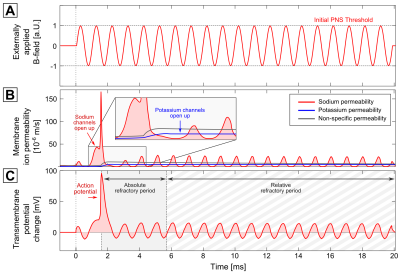
Figure 2 shows an exemplary stimulation of the fiber shown in Fig. 1 using the 1 kHz sinusoidal imaging gradient, whose amplitude is normalized to 1 at threshold (A). After 1.5 ms, the sodium permeability increases (B), leading to a strong current (causing the action potential and thus PNS, C). Following this activation of sodium channels, the potassium and non-specific permeability (blue and grey curve) increase to re-establish the resting state. As long as the potassium/non-specific permeability is elevated no action potential can be evoked (the “absolute refractory period”) or only at a higher stimulation threshold (the “relative refractory period”).
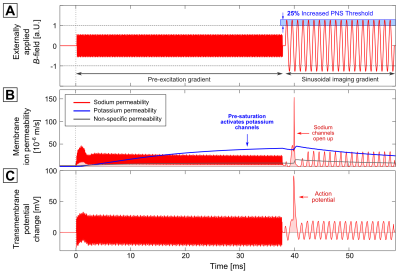
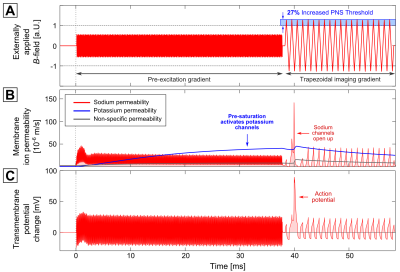
Figure 3 shows the same 1 kHz sinusoidal imaging gradient following a 10 kHz pre-excitation gradient with 38 ms in duration (in Fig. 4, a trapezoidal imaging gradient was used instead). The pre-excitation affects the sodium channels (second row, red curve), but is not strong enough to evoke an AP (the sodium permeability oscillates around a constant value after 2.5 ms). The potassium permeability, however, increases steadily throughout the entire pre-excitation gradient. This potassium current decreases neuronal excitability (i.e., it has an inhibitory effect on AP generation). As a consequence, the stimulation threshold for the following imaging gradient increases by 25%.
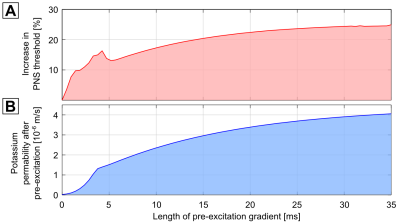
Figure 5 shows the increase in PNS threshold as a function of the duration of the pre-excitation gradient showing a plateau of about 25% for pre-excitation durations greater than 30 ms. Nerve pre-excitation waveforms with higher threshold reductions might be possible through further optimization. Here we only optimized the amplitude and frequency using a simplex optimization.
Conclusion
We used a mammalian nerve dynamic model to assess PNS reduction from a short pre-excitation gradient waveform prior to the imaging gradients. The method uses high frequency waveforms to activate potassium channels, which reduces neuronal excitability. The model predicts a substantial increase in PNS threshold with this method, but further experiments are needed to optimize and fully validate the method.Acknowledgements
NIH grants: K99/R00 EB019482, P41EB015896, U01EB025121, U01EB025162References
[1] McIntyre et al., "Modeling the excitability
of mammalian nerve fibers: Influence of afterpotentials on the recovery
cycle", J Neurophysiol. 87(2), 2002
[2] Davids et al., “Modeling of Peripheral Nervous Stimulation Thresholds in Realistic Body Models”, Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, Hawaii, USA, 2017
[3] Davids et al., “Predicting magnetostimulation thresholds in the peripheral nervous system using realistic body models”, Sci. Rep. 7:5316, 2017
[4] Davids et al., “Simulation of Peripheral Nerve Stimulation Thresholds of MRI Gradient Coils”, Proceedings of the 26th Annual Meeting of ISMRM, Paris, France, 2018
[5] Kapural et al., “Comparison of 10-kHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-Month Results From a Multicenter, Randomized, Controlled Pivotal Trial”, Neurosurgery. 79(5):667-677, 2016
[6] Avendaño-Coy et al., “Effect of Unmodulated 5-kHz Alternating Currents Versus Transcutaneous Electrical Nerve Stimulation on Mechanical and Thermal Pain, Tactile Threshold, and Peripheral Nerve Conduction: A Double-Blind, Placebo-Controlled Crossover Trial”, Arch Phys Med Rehabil. 98(5):888-895, 2017
[7] Rattay et al., “Analysis of Models for External Stimulation of Axons” IEEE Transactions on Biomedical Engineering, 33, 974-977, 1986
Figures