0287
High resolution in-vivo diffusion tensor cardiovascular magnetic resonance: a comparison of single-shot EPI and interleaved spiral trajectories with motion induced phase correction1Cardiovascular Magnetic Resonance Unit, Royal Brompton Hospital, London, United Kingdom, 2National Heart and Lung Institute, Imperial College, London, United Kingdom, 3National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, United States
Synopsis
The spatial resolution of DT-CMR STEAM acquisitions was increased by implementing an interleaved variable density spiral readout. Bulk motion during STEAM diffusion encoding is unavoidably encoded in the image phase which can result in signal loss for multi-shot acquisition when the multiple interleaves are combined. A phase correction was implemented using the fully sampled centres of k-space to calculate the differences in phase between interleaves. In 7 volunteers we show improved data quality at 2.0x2.0mm2 using interleaved spirals compared to single-shot EPI and we obtain similar DT-CMR parameters.
Introduction:
While diffusion tensor cardiovascular magnetic resonance (DT-CMR) provides myocardial microstructural information, spatial resolution is typically limited. Increasing the spatial resolution of single-shot STEAM EPI requires a longer readout which results in artefacts due to T2* and off-resonance. Interleaved acquisitions are frequently used to increase spatial resolution in CMR, but bulk motion is encoded in the image phase of STEAM DT-CMR acquisitions, leading to signal loss artefacts when interleaves are combined in complex space. In recent work, a spiral sequence was shown to reproduce the DT-CMR parameters of an established EPI technique (1, 2) at the same resolution with a higher signal-to-noise-ratio (SNR). In this work the resolution is increased using interleaved variable density spirals with a novel correction for the motion induced phase.Methods:
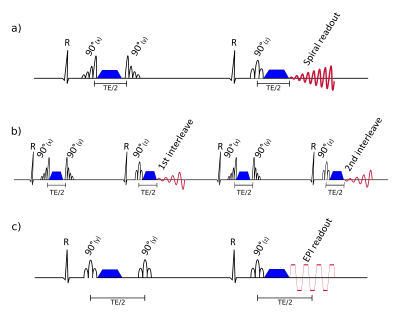
A spiral STEAM sequence (1) with a 2D reduced field-of-view (FOV) and asymmetric RF-pulses was modified to use interleaved variable-density spirals acquired over 4 cardiac cycles (figure 1a/b). Data were acquired on a Siemens Skyra 3T scanner in 7 healthy volunteers 27 [19-53] years, in a single mid-ventricular slice in systole with both spiral sequences (resolution: 2.8x2.8x8mm3 with single interleave spiral and 2.0x2.0x8mm3 with two interleaved spirals, TE=11ms) and a single-shot EPI sequence (resolution: 2.8x2.8x8mm3 and 2.0x2.0x8mm3 with TE=24ms and TE=34ms respectively) (figure 1c). Each EPI breath hold was 18 cardiac cycles long and acquired 1 average of each of 6 diffusion weighted images. Each spiral breath hold was 16 cardiac cycles and acquired 1 average of either 6 diffusion weighted images for the single shot acquisition or 3 diffusion weighted images for the interleaved acquisition. For each sequence 10 averages of bmain=600s/mm2 (20 breath-holds interleaved-spiral/10 breath-holds single shot acquisitions) and 1 average bref=150s/mm2 (2/1 breath-holds) in each of 6 diffusion directions were acquired.
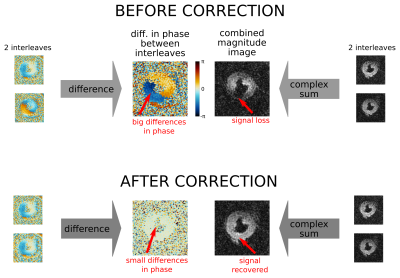
The spiral images were reconstructed off-line and, for the interleaved acquisitions, a phase correction was performed (3-5) to correct for motion induced phase differences between the interleaves. Both interleaves corresponding to each image were windowed in k-space (0.25x field of view Gaussian) leaving the low-frequency motion-induced phase data. This was used to match the phase of the second interleave to that of the first (figure 2). The multiple receive coil spiral data was combined using an optimal SNR coil-weighted reconstruction (6). For the EPI sequence a product SENSE x2 reconstruction was used.
The diffusion tensor (DT) was
calculated pixel-wise with in-house MATLAB software (7)
using all averages for all sequences (matched number of averages) and then with
5 averages of bmain=600s/mm2 and 1 average and 3
diffusion directions of bref=150s/mm2 for the interleaved
spiral sequence to match the number of breath-holds to the single shot
spiral/EPI acquisitions.
Results:
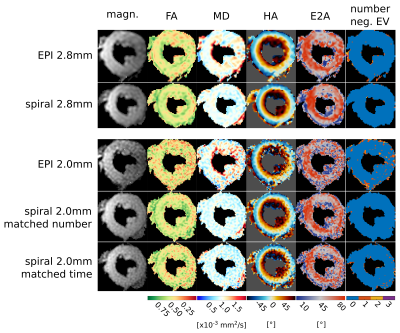
Example raw images, DT parameter maps and negative eigenvalue maps are shown in figure 3 and the subject-wise comparison of DT parameters in figure 4. There was no significant difference (p>0.05) in MD, FA and E2A between the standard (2.8x2.8mm2) and either of the high resolution (2.0x2.0mm2) spiral acquisitions. However, there were significant differences for EPI measurements of FA and E2A between the two resolutions. A comparison of the data quality and the SNR is shown in figure 5. The data quality measures are significantly reduced when increasing the resolution of the EPI acquisition, which is suggestive of poorer quality data at higher resolutions. There is no significant difference between the data quality measures acquired with the standard resolution or either of the high resolution spiral reconstructions.Discussion:
To enable inter-interleaf motion correction, a variable density spiral readout was used and the fully sampled centre of k-space was used to correct for differences in motion-induced phase between the two interleaves combined in each image. This is the first demonstration of a segmented STEAM in DT-CMR and the first to use phase correction for interleaved DT-CMR. The interleaved sequence demonstrates better agreement between DT-CMR parameters when comparing the high and standard resolution acquisitions than the EPI sequence. The data quality measured via stdTA and %neg.EV was similar between resolutions for the spiral sequences but showed significant reductions for the high-resolution EPI acquisition. In future work the image quality may be further improved by an implementation of an off-resonance correction or parallel imaging to further increase the resolution.Conclusion:
For high-resolution DT-CMR, the phase-corrected interleaved variable-density spiral readout provides higher SNR and a lower %neg.EV than the equivalent single-shot EPI readout while reproducing the DT-CMR parameters. This spiral technique is therefore a promising method for high resolution DT-CMR and could support future work to image patient cohorts with a thin myocardium or to get further insight into the structure and function of the thin wall of the right ventricle.Acknowledgements
This work was founded by heart research uk, grant reference number RG2648/15/18References
1. Gorodezky M, Ferreira P, Scott AD, Nielles-Vallespin S, Pennell D, Firmin D. A comparison of spiral and EPI trajectories for in-vivo cardiac DTI. Society for Cardiovascular Magnetic Resonance 20th Annual Scientific Sessions; 2017; Washington DC, USA.
2. Gorodezky M, Scott AD, Ferreira P, Nielles-Vallespin S, Khalique Z, Pennell D, Firmin D. In-vivo comparison of STEAM EPI and STEAM spiral diffusion-weighted sequences. International Society for Magnetic Resonance in Medicine Annual Meeting; 2017; Honolulu, USA.
3. Scott AD, Nielles‐Vallespin S, Ferreira PF, McGill LA, Pennell DJ, Firmin DN. The effects of noise in cardiac diffusion tensor imaging and the benefits of averaging complex data. NMR in Biomedicine. 2016;29(5):588-99.
4. Pipe JG, Farthing VG, Forbes KP. Multishot diffusion‐weighted FSE using PROPELLER MRI. Magnetic resonance in medicine. 2002;47(1):42-52.
5. Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magnetic resonance in medicine. 1999;42(5):963-9.
6. Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller O. The NMR phased array. Magnetic resonance in medicine. 1990;16(2):192-225.
7. Ferreira PF, Kilner PJ, McGill L-A, Nielles-Vallespin S, Scott AD, Ho SY, et al. In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobility in hypertrophic cardiomyopathy. Journal of Cardiovascular Magnetic Resonance. 2014;16(1):1.
Figures