0286
Cardiac Phase-resolved Late-Gadolinium Enhancement Imaging1Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3Computer Assisted Clincial Medicine, Heidelberg University, Mannheim, Germany, 4University of Minnesota, Minneapolis, MN, United States, 5Working Group on Cardiovascular Magnetic Resonance Imaging, Max-Delbrück-Centrum and Charité -Medical University Berlin, Berlin, Germany, 6Department of Cardiology and Nephrology, HELIOS Klinikum Berlin-Buch, Berlin, Germany
Synopsis
Late Gadolinium Enhancement (LGE) is commonly acquired during a single end-diastolic phase with inversion-recovery contrast that nulls healthy myocardial tissue. In this work, we propose a method for acquisition of cardiac phase-resolved LGE images based on an ECG triggered Look-Locker experiment with continuous FLASH imaging. Semi-quantitative evaluation of this pulsed-inversion recovery allows synthetization of LGE image contrast for all cardiac phases. Accurate functional depiction with temporal resolution up to 60 ms is obtained in healthy subjects at 3T. Images of 20 patients on a clinical 1.5T scanner show promising depiction of focal scar at a temporal resolution of 80ms.
Introduction
Late Gadolinium Enhancement (LGE) imaging is clinically established as gold-standard for characterization of scar and focal fibrosis. In LGE inversion-recovery imaging is used after contrast agent administration to depict contrast agent retention. However, in order to obtain the desired contrast that nulls the healthy myocardium, imaging is commonly restricted to a single cardiac phase.
In this study, we develop a method for acquisition of cardiac phase-resolved LGE images. We extend a recently proposed dynamic T1 mapping approach1 to semi-quantitative imaging after contrast administration, which allows for retrospective synthesization of LGE contrast for all cardiac phases.
Methods
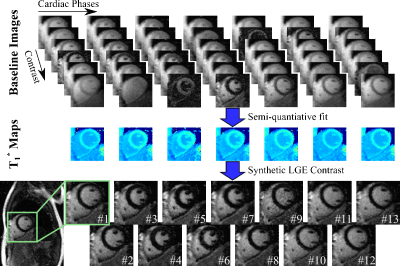
Sequence: The proposed sequence employs multiple ECG-triggered Look-Locker experiments (Fig. 1). First, the magnetization is driven to steady-state. Following an inversion, FLASH readouts are continuously performed, acquiring k-space data in a segmented manner. This pulsed-recovery curve spans multiple (typically 2) heart-beats, enabling the acquisition of multiple inversion times per cardiac phase. Upon re-reaching the steady-state the magnetization is re-inverted, and the next k-space segment is acquired. After completion of these segments, the entire experiment is repeated by varying the inversion pulse position throughout the cardiac cycle, in order to finely sample the recovery. Semi-quantiative information is then obtained using a 2-parameter model (S=A(1–2exp(-t/T1*)) and used on a per-voxel basis to generate LGE contrast as SLGE=A(1–2exp(-TI/T1*)). Here TI is a retrospectively chosen inversion time that is the same for all cardiac phases.
In Vivo Imaging: 4 healthy subjects (39±17years, 3males) were scanned on a 3T scanner (Siemens Prisma), with the following image parameters: TR/TE/FA=5/2.6ms/3°, FOV/resolution=300x225/1.9x1.9mm2, GRAPPA=2, slice=10mm, water-selective excitation, temp. resolution=60ms, breath-hold=17-19s. Additionally, 20 patients (50±16years, 13males, indications: suspected CAD:6, known CAD:5, myocarditis:7, DCM:1, HCM:1) were imaged on a clinical 1.5T scanner (Siemens Avanto Fit). Due to decreased gradient performance and lower baseline SNR, following sequence parameters were adopted at 1.5T: TR/TE/FA=6.7/3.2ms/6°, resolution=2.1x2.1mm2, temp. resolution=80ms, breath-hold=15-18s.
Results
Figure 2 illustrates image synthesization in a healthy subject at 3T. Despite wide variations in the baseline image contrast, a consistent T1* value is obtained across the cardiac cycle. This leads to robust nulling of the healthy myocardium for all phases of the synthesized LGE images.
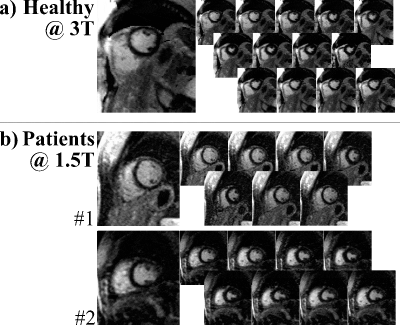
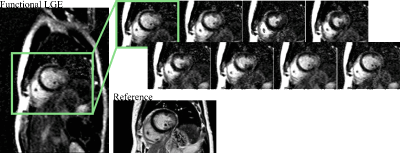
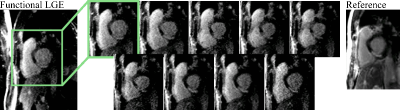
Figure 3a shows images acquired at 3T, depicting sharp delineation of the myocardium against blood pools without visually apparent temporal blurring. Patient images at 1.5T show thorough nulling of healthy myocardium throughout the cardiac cycle (Fig. 3b), despite lower baseline SNR, while simultaneously representing the myocardial contractility. Across the patient population, 20 images were of diagnostic image quality, while three cases suffered from fold-over artifacts due to sub-optimal FOV adjustment. One case exhibited two corrupted phases, due to sustained arrhythmia. Images of patients with known CAD show clear enhancement of the infarcted area (Fig. 4 lateral, Fig. 5 antero-septal). Retrospective choice of the inversion time allows for optimized scar-myocardium and scar-blood contrast, allowing good delineation against both. Additionally, the phase-resolved images depict scar deformation throughout the heart-beat.
Discussion
In this work, functional LGE imaging was enabled by semi-quantitative assessment of the longitudinal relaxation time in a phase-resolved manner. As previously proposed, quantitative T1 information can be used on a per-voxel basis to synthesize LGE images2 that provide accurate quantification of scar area3. Retrospective adjustment of the LGE contrast may even circumvent the problem of incomplete myocardial nulling in the presence of incorrect inversion times, as commonly observed in magnitude based LGE images.
Cross-comparison of end-diastolic LGE images with other cardiac phases has been shown to improve diagnostic confidence4, especially for assessing scar transmurality5. Confounding depiction of scar in standard LGE imaging may also occur when a short-axis slice is adversely placed to intersect with the right ventricular blood-pool causing an apparent myocardial hyper-enhancement. While LGE images can be acquired at multiple phases in different heart-beats, this requires long scan times, and image comparison is hampered by differential breath-holding. The proposed technique improves upon these shortcomings by efficiently assessing myocardial viability through the entire cardiac cycle.
The proposed technique may also be used to simultaneously evaluate cardiac function and tissue viability in a single scan, if sufficient temporal resolution is ensured. Advanced regularizations to improve spatio-temporal resolution6 are currently being investigated and warrant further research.
Conclusion
The proposed method for cardiac phase-resolved LGE imaging enables assessment of scar and focal fibrosis through the cardiac cycle. Excellent blood-myocardium contrast at a temporal-resolution of 60 ms is achieved at 3T. Patient images at 1.5T show clear depiction of the scar, while simultaneously displaying myocardial contraction and providing visualization of scar displacement.Acknowledgements
Grant support: NIH R00HL111410, NIH P41EB015894 and NSF CCF-1651825.References
- Weingärtner S, Shenoy C, Rieger B, Schad LR, Schulz-Menger J, Akçakaya M. Temporally resolved parametric assessment of Z-magnetization recovery (TOPAZ): Dynamic myocardial T1 mapping using a cine steady-state look-locker approach. Magn Reson Med. 2017;doi:10.1002/mrm.26887
- Varga-Szemes A, van der Geest RJ, Spottiswoode BS, Suranyi P, Ruzsics B, De Cecco CN, Muscogiuri G, Cannaò PM, Fox MA, Wichmann JL, Vliegenthart R, Schoepf UJ. Myocardial Late Gadolinium Enhancement: Accuracy of T1 Mapping-based Synthetic Inversion-Recovery Imaging. Radiology. 2016;278(2):374-82
- Varga-Szemes A, van der Geest RJ2 Schoepf UJ, Spottiswoode BS, De Cecco CN, Muscogiuri G, Wichmann JL, Mangold S, Fuller SR, Maurovich-Horvat P, Merkely B, Litwin SE, Vliegenthart R, Suranyi P. Effect of inversion time on the precision of myocardial late gadolinium enhancement quantification evaluated with synthetic inversion recovery MR imaging. Eur Radiol. 217;27(8):3235-43
- Schuster A, Chiribiri A, Ishida M, Morton G, Paul M, Hussain S, Bigalke B, Perera D, Nagel E. End-systolic versus end-diastolic late gadolinium enhanced imaging for the assessment of scar transmurality. Int J Cardiovasc Imaging. 2012;28(4):773-81
- Matsumoto H, Matsuda T, Miyamoto K, Shimada T, Hayashi A, Mikuri M, Hiraoka Y. Late gadolinium-enhanced cardiovascular MRI at end-systole: feasibility study. AJR Am J Roentgenol. 2010;195(5):1088-94
- Moeller S, Weingartner S, Akcakaya M. Multi-scale locally low-rank noise reduction for high-resolution dynamic quantitative cardiac MRI. Conf Proc IEEE Eng Med Biol Soc. 2017 Jul;2017:1473-1476.
Figures