0285
Cardiac Magnetic Resonance Elastography for the Diagnosis of Patients with Heart Failure with Preserved Ejection FractionArvin Arani1, Shivaram P. Arunachalam1, Phillip J. Rossman1, Joshua D. Trzasko1, Kevin Glaser1, Yi Sui1, Kiaran McGee1, Armando Manduca1, Barry A. Borlaug1, Richard Ehman1, and Philip Araoz1
1Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
Heart failure with preserved ejection fraction (HFpEF) accounts for half of incident heart failure cases per year. Currently, the diagnostic reference standard is invasive. The objective of this study is to evaluate if cardiac MR elastography (MRE) can measure increased myocardial stiffness in patients with HFpEF. Fifty-eight volunteers and 10 patients were enrolled. The mean left-ventricle myocardial stiffness of HFpEF patients (10.5±1.7 kPa) was significantly higher (p=0.002) than control subjects (8.0±1.2 kPa).This study motivates further investigation into the use of cardiac MRE as a quantitative noninvasive imaging technique to assist in the diagnosis and therapy monitoring of patients with HFpEF.
Introduction
Heart failure incidence is increasing, now reaching “epidemic” proportions (1). Heart failure with preserved ejection fraction (HFpEF) accounts for half of incident heart failure cases (2), disproportionately affects women (3), portends a 30% one year mortality rate (4), and is responsible for an estimated $18 billion in overall medical costs per year (5). HFpEF has been called “one of the greatest unmet needs in Cardiology” (1). Despite over twelve, large, randomized treatment trials of HFpEF, none have shown a survival benefit (6). Currently, the diagnostic reference standard is hemodynamic catheterization, which detects increased left-ventricular (LV) filling pressure. However, catheterization is invasive and cannot be widely applied for HFpEF diagnosis. One hypothesis is that elevated pressures are due to increased stiffness of the myocardium in patients with HFpEF. Recently, a high-frequency cardiac MR elastography (MRE) technique was developed that is capable of measuring myocardial stiffness noninvasively (7,8). The objective of this abstract was to evaluate if cardiac MRE can detect increased myocardial stiffness in patients with HFpEF.Methods
Fifty-eight volunteers and 10 patients with HFpEF were enrolled in this study, after IRB approval and obtaining formal written consent. One volunteer and 5 patients were excluded from the study due to poor image quality as determined by a quantitative octahedral shear strain signal-to-noise ratio metric (9). All subjects were imaged feet first in the supine position in a 1.5-Tesla, wide-bore, MR imager (Optima MR450W; GE Healthcare, WI, USA). Cardiac MRE was used to measure myocardial stiffness in the left ventricle of each subject. The experimental setup is shown in Figure 1. A flow-compensated, cardiac-gated, spin-echo, single-shot, echo-planar imaging, MRE sequence was used with 140-Hz vibrations and a TR matched to each subject’s heart rate with ECG gating. Other imaging parameters included TE = 69ms; FOV = 32 cm; 64x64 acquisition matrix; parallel imaging acceleration factor = 2; 5 contiguous 5-mm-thick axial slices; 2 motion-encoding gradient (MEG) pairs on each side of the refocusing pulse matched to the vibration frequency; alternating x, y, z, and 0 MEG directions; and 4 phase offsets spaced evenly over one vibration period. For image processing purposes, the image matrix was reformatted to 256x256x20 to give an isotropic voxel size of 1.25 mm. Images were acquired at the minimum delay possible in the cardiac cycle (~100 ms) after the R-wave ECG trigger. A 3D local-frequency estimation algorithm was used to calculate the stiffness, which we defined to be the tissue density (assumed to be 1 g/cm3) times the square of the shear wave speed (i.e., shear wavelength times the motion frequency). Statistical analysis was done using a Mann-Whitney U test of significance using a commercial software package (OriginPro 2015, OriginLab Corporation, Northampton, MA) (10). A p-value of less than 0.05 was considered statistically significant.Results
The left-ventricle myocardial stiffness of the 5 HFpEF patients (mean stiffness: 10.5 ± 1.7 kPa; age (years): mean = 62.4, range = 54-74, median = 65) was significantly higher (p = 0.002) than the 57 normal control subjects (mean stiffness: 8.0 ± 1.2 kPa; age (years): mean = 33.5, range: 18-87, median = 27), as shown in Figure 2. A typical short-axis, left-ventricle, stiffness map from a healthy volunteer and a patient with HFpEF are shown in Figure 3. The images show the global change in myocardial stiffness that occurs in these patients.Conclusions
This study demonstrates the feasibility of 3D, high-frequency, cardiac MRE as a contrast-agent-free diagnostic imaging technique for quantitatively measuring myocardial stiffness in patients with heart failure with preserved ejection fraction. The positive outcomes of this study motivate further investigation into the use of cardiac MRE as a quantitative, noninvasive, and exogenous contrast-free imaging technique to assist in the diagnosis and therapy monitoring of patients with HFpEF.Acknowledgements
This study was supported by National Institutes of Health (NIH) grants 5R01HL115144 and EB001981 and the American Heart Association - Midwest Affiliate. (16POST29900011).References
1. Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail 2011;13(1):18-28. 2. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129(3):399-410. 3. Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA internal medicine 2015;175(6):996-1004. 4. Ferrari R, Bohm M, Cleland JG, Paulus WJ, Pieske B, Rapezzi C, Tavazzi L. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail 2015;17(7):665-671. 5. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010;121(7):e46-e215. 6. Nordlund D, Kanski M, Jablonowski R, Koul S, Erlinge D, Carlsson M, Engblom H, Aletras AH, Arheden H. Experimental validation of contrast-enhanced SSFP cine CMR for quantification of myocardium at risk in acute myocardial infarction. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 2017;19(1):12. 7. Arani A, Arunachalam SP, Chang ICY, Baffour F, Rossman PJ, Glaser KJ, Trzasko JD, McGee KP, Manduca A, Grogan M, Dispenzieri A, Ehman RL, Araoz PA. Cardiac MR elastography for quantitative assessment of elevated myocardial stiffness in cardiac amyloidosis. Journal of magnetic resonance imaging : JMRI 2017;46(5):1361-1367. 8. Arani A, Glaser KL, Arunachalam SP, Rossman PJ, Lake DS, Trzasko JD, Manduca A, McGee KP, Ehman RL, Araoz PA. In vivo, high-frequency three-dimensional cardiac MR elastography: Feasibility in normal volunteers. Magnet Reson Med 2017;77(1):351-360. 9. McGarry MD, Van Houten EE, Perrinez PR, Pattison AJ, Weaver JB, Paulsen KD. An octahedral shear strain-based measure of SNR for 3D MR elastography. Physics in medicine and biology 2011;56(13):N153-164. 10. Siegel S. Nonparametric statistics for the behavioral sciences. 1956.Figures
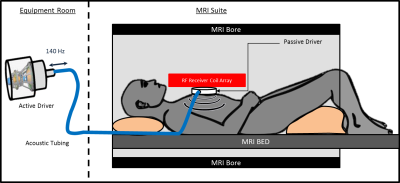
Figure 1: Cardiac MRE experimental setup.
An active driver delivers 140-Hz vibrations through tubing to a passive driver
that is strapped to the subject’s chest. Shear waves are transmitted into the
myocardium and the wave displacement field is imaged with MRE.
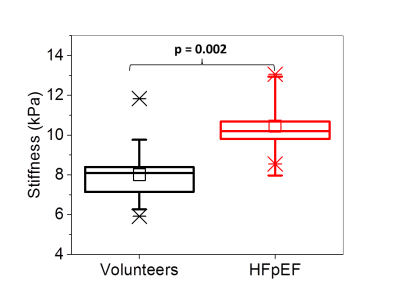
Figure
2:
Increased myocardial stiffness in patients with HFpEF (n=5) compared to normal,
healthy volunteers (n=57). The top and bottom of the box represent the 1st
and 3rd quartiles, the square represents the group mean, and the
line represents the median. The whiskers represent 1 standard deviation from
the mean.
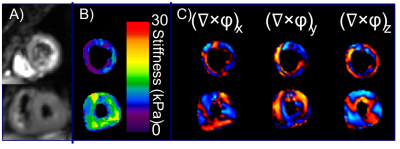
Figure
3: Short-axis MRE magnitude images (A), stiffness
maps (B), and wave images in all three directions (C) for a healthy female (top
row) and a female patient with HFpEF (bottom row).