0281
Detection of D-amino acid oxidase using hyperpolarized molecular probes1Laboratory for Functional and Metabolic Imaging (LIFMET), EPFL, Lausanne, Switzerland, 2Center for Biomedical Imaging (CIBM), Lausanne, Switzerland
Synopsis
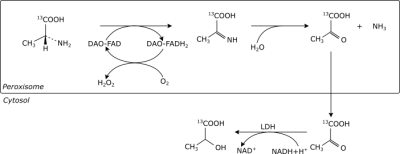
D-amino acid oxidase (DAO) is an enzyme that catalyzes the degradation of D-amino acids in the body. Here, we explored the possibility of detecting D-amino acid oxidase activity by monitoring its metabolism in the rat kidney after a bolus injection of hyperpolarized D-[1-13C]alanine. Our data show that D-alanine is readily converted to lactate only when the DAO enzyme is not inhibited, indicating that the observed metabolism is that of DAO.
Introduction
An increasing number of studies recognizes D-amino acids and as biomarkers of kidney and brain diseases1,2. Their levels in tissues and blood stream are mainly regulated by the enzyme D-amino acid oxidase (DAO), whose function is to promote their degradation by catalyzing oxidative deamination and producing the corresponding 2-oxo acid. DAO is located in the peroxisome and is most abundant in the kidneys, but it’s also found in the brain and liver of most mammals. The activity of this enzyme is traditionally detected by measuring in vitro its reaction products with chromatographic or colorimetric methods. The present study aims to assess the feasibility of exploiting the high sensitivity given by dynamic nuclear polarization to detect rat kidney DAO activity in a minimally invasive way using hyperpolarized D-[1-13C]alanine.Methods
D-Amino Acid oxidase activity was assessed monitoring the D-[1-13C]alanine metabolism in the rat kidney before and after DAO inhibitor injection. Experiments where the L enantiomer was infused were performed as well, as a control for any change in kidney metabolism.
Hyperpolarization: samples of 5 M of D-[1-13C]alanine and L-[1-13C]alanine doped with 25 mM of OX063 radical were polarized in a custom-built 7 T polarizer at 1 K by shining microwave radiation at 196.59 GHz, 50 mW in power. The polarization buildup was monitored for approximately 2 hours, after which the samples were rapidly dissolved in 5.5 mL of a hot buffer solution and transferred (3 s) to a separator infusion pump located in a 9.4 T/ 31 cm animal scanner.
Animals: male Wistar rats were anaesthetized using 1-2% isoflurane. The hyperpolarized solution was administered through the femoral vein. Blood gases, pH and physiological parameters were monitored shortly after each infusion.
DAO inhibitor: a dose of 20 mg/kg of a neutralized 4H-Furo[3,2-b]pyrrole-5-carboxylic acid (SAN) solution3 was injected intravenously in the rat 20 minutes prior to the HP [1-13C]alanine infusion.
Acquisition: 1.2 mL of the hyperpolarized alanine solution was injected over 9 s. The concentration of the L-alanine bolus prior to infusion was ≈20 mM, while two different D-alanine bolus concentrations were considered – 20 mM and 40 mM, to evaluate the possibility of enzyme/transporter saturation. The 13C signal acquisition was performed with respiration and cardiac gating. 1H-decoupled 13C FIDs were acquired by applying 30° BIR4 pulses using a single loop 1H / quadrature 13C surface coil placed over the left kidney of the animal. The repetition time between each scan was ≈ 3 s. A non-hyperpolarized baseline scan was acquired after each hyperpolarized experiment to assess the natural abundance 13C fat contributions to the spectrum.
Data
analysis: for each infusion, the acquired FIDs were summed and the resulting
spectrum was fitted with Bayes (Washington University, St. Louis) to determine
the peak amplitudes and the ratios between metabolites.
Results and discussion
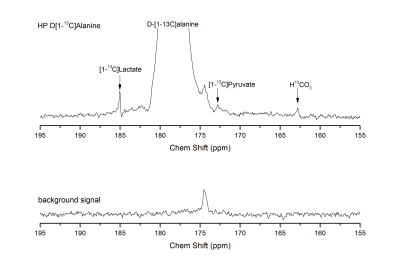
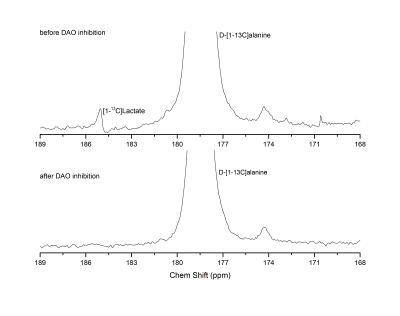
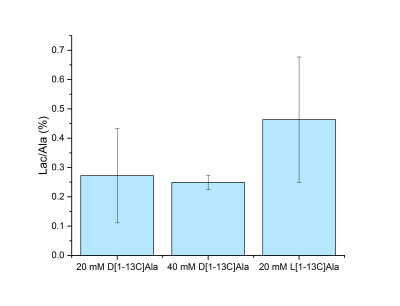
D-[1-13C]alanine metabolic products were successfully observed (Fig. 2), with lactate being the dominant one. Pyruvate and HCO3- could not be consistently detected when injecting the 20 mM bolus, while they became more apparent upon dosage increase. Those metabolites were not observed when the DAO inhibitor was injected prior to alanine infusion (Fig. 3), indicating a requirement for DAO enzymatic activity. Quantification of the metabolite ratios is made difficult by the overall low SNR of the experiments and consistent results could only be obtained for the lactate/alanine ratio of the high dose experiments. No significant difference could however be observed in the lactate/alanine ratios when comparing different dosages, suggesting that the concentration of the injected precursor is not high enough to cause enzymatic/transport saturation. Control HP L-[1-13C]alanine infusion experiments showed slightly higher lactate conversion (Fig. 4). The SAN inhibitor was observed to cause no change in the conversion of L-[1-13C]alanine to lactate (data not shown).Conclusions
D-Amino acid oxidase activity could be detected in the rat kidney with the conversion of HP D-[1-13C]alanine to lactate consistently observed. These results demonstrate that peroxisomal metabolism can be monitored in real time using this method.Acknowledgements
This work was supported by the Swiss State Secretariat for Education, Research and Innovation (SERI) within the Marie Curie Initial Training Network EUROPOL project (N° SERI: 15.0164), and by the Centre d'Imagerie Biomédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL and the Leenards and Jeantet Foundations.References
1. Ohide H. et al., Journal of Chromatography B, 879 (2011)
2. Kimura T. et al., Scientific Reports 6, (2016)
3. Sparey T. et al., Bioorganic & Medicinal Chemistry Letters, 18 (2008)
Figures